- Submissions

Full Text
Research in Medical & Engineering Sciences
The Efficacy of Sap Obtained from Commiphora gileadensis Against Cadmium-Induced Liver Toxicity in Rats
Abdulla S Aljohani1, Suliman Al Dakhil1, Amal S Soliman2*, Mohamed El-Alreshoodi3 and Ibrahim M El-Ashmawy1,4
1Department of Medical Biosciences, College of Veterinary Medicine, Qassim University, Buraidah, Qassim, Saudi Arabia
2High Institute of Engineering and Technology, Department of Basic Sciences, Alexandria, Egypt
3Salam Veterinary Group, Buraidah, Qassim, Saudi Arabia
4Department of Pharmacology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt
*Corresponding author:Amal S Soliman, Department of Basic Sciences, High Institute of Engineering and Technology, Alexandria, Egypt
Submission: September 24, 2025;Published: December 17, 2025

ISSN: 2576-8816Volume12 Issue 2
Abstract
Cadmium (Cd), a toxic heavy metal, ranks among the top ten environmental threats to human health, adversely affecting vital organs such as the kidneys, liver, pancreas, lungs, and testes. This study investigates the protective effects of Commiphora gileadensis (CG) sap against Cd-induced liver damage in male rats. Forty-two healthy adult male albino rats were randomly divided into seven groups: G1 (control), G2 (50mg/L cadmium chloride in drinking water), G3 and G4 (100mg/kg and 400mg/kg CG sap, respectively), G5 (50mg/L cadmium chloride + 100mg/kg CG sap), G6 (50mg/L cadmium chloride + 400mg/kg CG sap), and G7 (1% Tween 80, 1mL/rat as a vehicle). Cadmium exposure significantly reduced the antioxidant capacity and impaired liver functions. However, co-administration of CG sap markedly ameliorated these adverse effects, improving antioxidant capacity and restoring hepatic function. These findings suggest that CG sap has potential as a natural therapeutic agent to mitigate cadmium toxicity and its associated environmental hazards.
Keywords:Commiphora gileadensis; Cadmium; Liver functions; Oxidative stress; Antioxidants
Introduction
The livers are critical organs responsible for xenobiotic metabolism and storage, making them highly susceptible to damage from toxic substances. Cadmium (Cd), a non-essential transition metal, is a significant environmental contaminant known for its toxicity to humans and animals. Cd exposure occurs through industrial and agricultural activities, as well as contaminated food, water, and cigarette smoke [1,2]. Due to its long biological half-life (25-30 years), Cd bioaccumulates in tissues, primarily in the liver, causing severe oxidative stress and tissue damage [3].
Cd toxicity is associated with various health complications, including hepatic syndrome, characterized by impaired liver functions and increased morbidity and mortality. The toxic effects of Cd are largely mediated by its induction of reactive oxygen species (ROS), leading to oxidative stress, mitochondrial dysfunction, and damage to cellular components [4,5]. Antioxidants, both synthetic and natural, have been investigated for their ability to counteract Cd-induced oxidative damage. Among natural antioxidants, plant-derived compounds such as phenolics and flavonoids have shown significant protective effects [6].
Commiphora gileadensis (CG), commonly known as Balessan, is a medicinal plant native to arid regions, including Saudi Arabia. Traditionally, CG sap has been used for its anti-inflammatory, antimicrobial, and wound-healing properties [7,8]. Recent studies have highlighted the antioxidant potential of CG sap, which may offer protective effects against heavy metal toxicity [9,10]. This study evaluates the ameliorative effects of CG sap on Cd-induced liver damage in albino rats, with a focus on histopathological changes and biochemical markers.
Materials and Methods
Plant material
Commiphora gileadensis sap was collected by damaging the bark of the plant in the Medina region of Saudi Arabia.
In Vitro antioxidant activity assessment
One g of plant sap was extracted with 10 mL of ethanol using a magnetic stirrer at 1,000rpm (IKA, Staufen, Germany).
Total Phenolic Content (TPC)
TPC was estimated using the Folin-Ciocalteu method [11] with gallic acid as the standard.
Cupric Reducing Antioxidant Capacity (CUPRAC)
The CUPRAC assay was performed as reported by [12], using Trolox as the standard antioxidant. The experiment was conducted in triplicates, with values expressed as Trolox equivalents per gram of dry weight.
Total Flavonoid Content (TFC)
TFC was determined using the aluminum chloride colorimetric method [13]. Absorbance was measured at 405nm, with results expressed as quercetin equivalents (QE).
Ferric Reducing/Antioxidant Power (FRAP)
The FRAP assay was conducted following the method of [14], using Trolox as the standard.
DPPH radical-scavenging activity
The DPPH assay was carried out using the method of [15], with results measured at 517nm.
ABTS radical-scavenging activity
The ABTS assay was performed as described by [16], with results expressed at 734nm.
Median Lethal Dose (LD50)
The LD50 of CG sap was determined using 12 male Swiss albino mice (20-25g). The mice were divided into four groups (n=3 per group) and acclimated for seven days. Doses of 1.0, 2.5, and 5.0g/ kg body weight of CG sap in 1% Tween 80 were administered orally. Observations for mortality and behavioral changes were conducted for 48 hours [17].
Animal Experimentation
Forty-two adult male albino rats (200-220g) were obtained from the Faculty of Pharmacy, King Saud University, Riyadh, Saudi Arabia. After a two-week acclimation period, the rats were randomly divided into seven groups (n=6 per group) as follows: Control Group (G1; NC): Normal drinking water and standard diet. Positive Control (G2: Cd): 50mg/L cadmium chloride in drinking water. Experimental Groups (G3: CG1, G4: CG4): 100mg/kg and 400mg/kg CG sap orally. Combination Groups (G5, G6): 50mg/L cadmium chloride + 100mg/kg or 400mg/kg CG sap orally. Vehicle Control (G7): 1mL of 1% Tween 80 orally.
All treatments were administered daily for 60 days. Cadmium chloride was supplied via drinking water, while CG sap and vehicle were administered orally by gavage [18].
Blood sampling and histopathological examination
At the end of the experimental period, blood samples were collected under light ether anesthesia for serum preparation to measure biochemical parameters using commercial kits. Liver tissues were harvested, preserved in 10% buffered formalin, and processed for histopathological analysis.
Statistical analysis
Results were reported as mean ± SEM (Standard Error of Mean). To assess the influence of the seven treatment groups on the different biochemical parameters, one- way analysis of variance (ANOVA) by Tukey multiple tests as post hoc test were used. The value of P<0.05 was used to indicate statistical significance. ALL Analyses were done using Statistical Package (IBM SPSS Statistics, version 26).
Result
The median lethal dose (LD50) of C. gileadensis sap
The findings indicated that none of the tested doses of Commiphora gileadensis, even at levels as high as 5000mg per kilogram of body weight, resulted in no fatalities within the various groups (Table 1).
Table 1:The number of Mortality of different groups of mice with different administered doses.

In vitro assessment of antioxidant activity
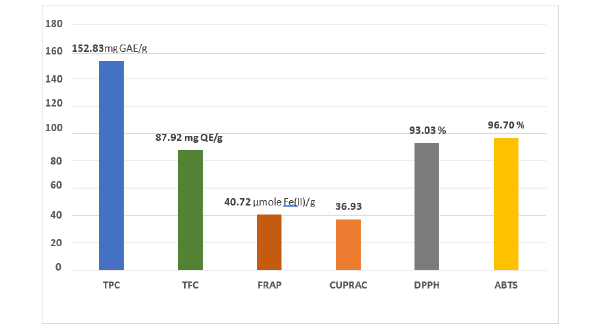
C. gileadensis had strong scavenging values depend on our result (Figure 1). The results showed that DPPH value of CG to be 75.27%, the ABTS value of CG was 98%, the TPC value of CG to be 101.47mg GAE/g and the TFC value of CG to be 77.13mg QE/g and the values of FRAP and CUPRAC were 40.72 and 36.93 respectively.
Figure 1:In vitro assessment of antioxidant activity {total phenolic content (TPC), cupric reducing antioxidant capacity (CUPRAC), total flavonoid content (TFC), ferric reducing/antioxidant power (FRAP), 2,2-diphenyl-1- picrylhydrazyl radical scavenging activity (DPPH), 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation assay (ABTS).
Effect of CG sap, and cadmium chloride on serum antioxidant concentration
Table 2:Effect of CG sap, and cadmium chloride on antioxidant concentration of CAT, MDA and total antioxidant.

NC (normal control): Cd (cadmium): CG1 (Commiphora gileadensis 100mg/kg b. wt.): CG4 (Commiphora gileadensis 400mg/kg b. wt.): Values within the same column carrying different letters are significantly different at P<0.05 based on Tukey post hoc multiple comparison test. Values are expressed as mean ± S.E. (N=6 rats).
Data observed that administration of C. gileadensis sap non significantly affected the serum level of catalase, total antioxidants and malondialdehyde (MDA) compared with the values of control groups (Table 2). The present data showed a significant (P<0.05) decreased in the serum level of catalase enzyme and total antioxidants in cadmium chloride treated group compared to all other groups of the study. While co- administration of C. gileadensis sap plus cadmium chloride significantly enhanced the values of serum levels of catalase enzyme; and total antioxidants and these values did not differ significantly with those of control groups. Administration of cadmium chloride significantly increased the serum level of MDA compared to other groups of the experiment. However, co-administration of C. gileadensis sap significantly decreased the MDA level but remained higher significantly than control values (Table 2).
Biochemical analysis
Biochemical analysis of various damage and function markers were analyzed. These markers include liver damage and function markers such as alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total protein (TP) and albumin (ALB). These tests aids determination of which part of the liver is damaged, and depending on the pattern of elevation, can assist organizing a differential diagnosis. Besides these markers, the kidney function markers such as the urea, creatinine and uric acid levels in the blood serum were investigated.
The liver function markers were analyzed including ALT, AST and ALP as shown in Table 3. As can be observed from Table, A significant increase in AST (305.11±15.03), ALP (261.16±8.35), and ALT (144.83±7.79) levels in the blood serum is clear in CD cohort which indicates the induction of liver injury. This reduction was alsoobserved even when the C. gileadensis dose to 100 and 400mg. The effect and reduction of liver function marker upon exposure to CD- C. gileadensis contributes to the potential protective effect of C. gileadensis against liver injury.
Table 3:The effect of different treatments on the serum levels of ALP, ALT and AST of rats intoxicated with CD for 8 weeks (values are expressed as mean ± SE.

NC (normal control): Cd (cadmium): CG1 (Commiphora gileadensis 100mg/kg b. wt.): CG4 (Commiphora gileadensis 400mg/kg b. wt.): Values within the same column carrying different letters are significantly different at P<0.05 based on Tukey post hoc multiple comparison test. Values are expressed as mean ± S.E. (N=6 rats).
Histopathological examination of liver in the different groups
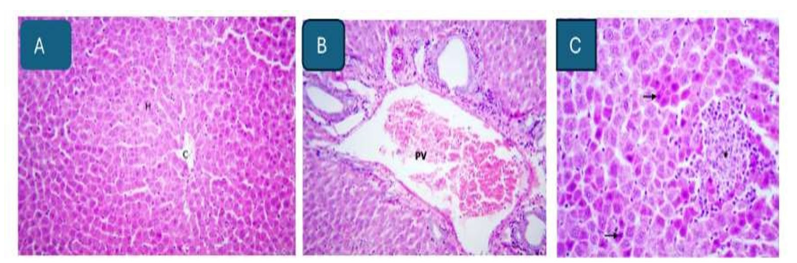
Control group: No histopathological changes were detected in the examined livers of these rats. Figure 2A revealed normal histological criteria.
Cadmium chloride group: The livers of the treated rats showed congestion and dilatation of central and portal veins (Figure 2B). focal aggregates of lymphocytes and macrophages, surrounded by coagulative necrosis of hepatocytes characterized by loss of cellular detail, individualized shrunken hepatocytes with hyper eosinophilic cytoplasm and pyknotic nuclei (Figure 2C).
Figure 2:A. Liver of control rat showing normal histological appearance of central vein (CV) and hepatocytes (H). H&E stainx200. B. Liver of rat administered 50mg cadmium chloride showing congestion and dilatation of portal vein (PV). H&E stainx200. C. Liver of rat administered 50mg cadmium chloride showing focal aggregates (asterisk) of lymphocytes and macrophages, surrounded by coagulative necrosis of hepatocytes characterized by loss of cellular detail, individualized shrunken hepatocytes with hyper eosinophilic cytoplasm and pyknotic nuclei (arrow). H&E stainx200.
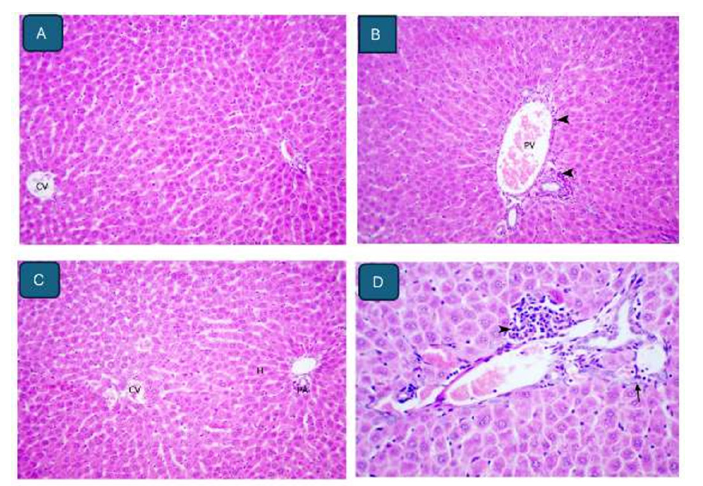
C. gileadensis sap 100mg/ kg b. wt. group: The liver showed normal histological appearance like control group. Occasionally, the liver showed mild congestion of central veins (Figure 3A).
C. gileadensis sap 400mg/ kg b. wt. group: The liver showed normal histological appearance like control group. Occasionally, the liver showed congestion of portal veins and mild lymphocytic infiltrate in portal areas (Figure 3B).
C. gileadensis sap 400mg/ kg b. wt. plus cadmium chloride group: The livers showed mild congestion of central veins with normal histological appearance of hepatocytes and portal areas (Figure 3C).
Figure 3:A. Liver of rat administered 100mg plant sap/kg b. wt. showing mild congestion of central veins (CV) and normal histological appearance of hepatocytes. H&E stain x 200. B. Liver of rat administered 400mg plant sap/kg b. wt. showing congestion of portal vein (PV) and mild lymphocytic (arrow) infiltrate in portal areas. H&E stain x 200. C. Liver of rat administered 400mg plant sap/kg b. wt. and 50mg cadmium chloride showing mild congestion of central veins (CV) with normal histological appearance of hepatocytes (H) and portal areas (PA). H&E stain x 200. D. Liver of rat administered 400mg plant sap/kg b. wt. and 50mg cadmium chloride showing mild biliary hyperplasia and small numbers of lymphocytes (arrowhead) and macrophages in portal areas. H&E stain x 400.
Discussion
This study is the first to explore the protective effects of Commiphora gileadensis (CG) sap against cadmium (Cd)- induced toxicity in liver tissues. Plants have large number of bioactive compounds with high antioxidant activity. Studies for the determination of the antioxidant activity of different plant species could contribute to revealing the value of these species as a source of new antioxidant compounds. Plants live in habitats with extreme environmental conditions (high temperatures, water stress, and high light irradiation in summer), for which they have developed different adaptive mechanisms, both morphological and physiological. One of these mechanisms is the prevention of oxidative stress, keeping ROS under dangerous levels [19], and using them for efficient signaling [20]. Secondary metabolites of plants, specifically phenols, can adjust the concentration of ROS, thus activating a network of biochemical events to increase tolerance, hence the importance of studying the antioxidant activity of typical plant species as C. gileadensis. The protective effects against oxidative stress are attributed to the natural antioxidants found in herbs and spices. These aromatic ingredients harbor compounds such as polyphenols, flavonoids, and phenolic compounds, which act as scavengers of free radicals, however antioxidants are substances that can scavenge free radicals and prevent the oxidative damage they generate in cells. In the present study, C. gileadensis sap had strong antioxidant activity by studying its DPPH, ABTS, FRAP and CUPRAC values. Some authors, confirmed the previous findings on some parts of CG other than its sap as leaves and bark. [21], reported the DPPH value of CG to be 75.27% which is lower than the value reported in this research. However, [22] reported higher values than in the present study. While, the ABTS value of CG to be 98% which is higher than the value reported in this research. [21] reported the TPC value of CG to be 101.47 mg GAE/g and the value was lower than the value reported in this research. [23] reported the TFC value of CG to be 77.13mg QE/g which is lower than the value reported in this research. However, there is no report in the literature of the values of FRAP and CUPRAC. The differences between results may be due to different parts used, extraction conditions such as methodology and solvents used in the extraction. The differences in results also, may be due to geographical locations of samples.
C. gileadensis sap is of wide safety margin as confirmed in the present study as LD50 was above 5g /kg b. wt. These results align with previous research conducted by [24] who reported that C. gileadensis sap safe up to 5g/kg b. wt. Consequently, any substance that demonstrates no harmful effects at a dosage of 5000mg/ kg is categorized as being ‘practically non-toxic.’ Based on these outcomes, it can be concluded that Commiphora gileadensis sap is regarded as safe and practically non- toxic [25]. Cadmium is one of the most toxic heavy metals and affects both animals and humans via induction of cellular oxidative stress and subsequent elevation of free radicals. Strong evidence suggests the harmful effect of cadmium. The livers are the organs with the highest concentration of cadmium present, after absorption [26]. When cadmium enters the body, it is transported into the bloodstream via erythrocytes and albumin and is then accumulated in liver and gut [27]. Cadmium excretion from the body is slow and occurs via the kidneys, urine, saliva, and milk during lactation. Cd exposure can result in a variety of adverse effects, such as renal and hepatic dysfunction, pulmonary edema, testicular damage, osteo-malacia, and damage to the adrenals and hemopoietic system [28]. It has been proved that after exposure, Cd enters the blood and binds to the erythrocyte membranes and blood albumin and then is transported to liver, where it bounds to metallothionein (MT) [28]. The Cd-MT complex is then released back into circulation [29]. and accumulates in the blood system, kidney, liver, lung, testis, brain, and bone [30].
In the current study, liver injury was evident in the Cd group, manifested by decreased total protein levels, increased liver enzymes, AST, ALT and ALP. These results are in agreement with earlier studies [31,32]. Recent reports have suggested that lysosome instability caused by Cd toxicity resulted in the leakage of hepatic enzymes, including ALT, ALP and AST, into the bloodstream [32]. CG sap is rich source of polyphenols. Polyphenols have been reported to possess a membrane stabilizing activity by inhibiting the generation of ROS induced by Cd and maintain the cell membrane structural integrity [33]. Our findings exhibited elevation in liver enzymes in Cd-treated group. These findings come in the same line with [34], who revealed that; Cd toxicity causing hepatic cell damage and its enzymes AST, ALP and ALT released into circulation, therefore the level of these enzymes in blood elevated than normal. Histological examination of liver tissue showed liver injury in the Cd-treated group, where hepatocytes lost their characteristic regular arrangement. Most hepatocytes exhibited vacuolation, and their nuclei were partially pyknotic. In contrast, CG a co- treatment showed hepatic-protective effects, proven by restoration of liver structure and function in these groups. The hepatic-protective effects of CG sap observed in this study, supporting the opinion that CG restored liver architecture and function following cadmium toxicity.
These histological abnormalities were found to be reduced in CG + Cd groups. The co- treatments with CG led to almost complete restoration of the Cd induced renal damage and restored the standard values of the kidney function markers.
Cd-induced hepatic dysfunction in this study was most likely due to an imbalance of oxidant/antioxidant capacity in the tissues. The present study showed a significant increase in MDA levels and a decrease in the activity of antioxidant enzymes, such as serum total antioxidants and CAT. The endogenous antioxidants are important scavengers of free radicals, which eventually lead to cellular dysfunction and death [6]. Our findings agreed with previous reports demonstrating that cadmium induces kidney toxicity by enhancing ROS formation and GSH consumption and inhibiting an antioxidant-mediated defense system [33]. Additionally, the nephroprotective effect of CG was owned to the improvement of renal oxidation and decrease of serum creatinine urea levels which are in consistence with [35]. In the present study, CG sap contains a high content of flavonoids. [36] studied one of the main actions of flavonoids, which enhanced renal function and diminished apoptotic and oxidative stress markers. Thus, the properties of CG may cause some of the Cd to be retained, preventing further adverse effects usually induced by toxic metals. In the current study, the observed Cd- induced hepatic histopathological abnormalities are consistent with previous results depicting similar alterations in rats treated with Cd administered as CdCl2. Most importantly, our study has shown that CG pretreatment possesses potent hepatic protective and antioxidative properties against cadmium toxicity.
Conclusion
Collectively, administration of cadmium chloride induces adverse effects on the liver functions and decreases the antioxidative status. Co-administration of CG sap with cadmium significantly mitigated the previous hazard effects by ameliorating the antioxidant status. The plant sap showed no signs of toxicity were observed during the acute toxicity study and the LD50 value indicates that sap is to be safe. Phytochemical investigation is also proposed to isolate the active fraction and eventually the pure compound.
Acknowledgement
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2024-9/1).
References
- Dharmadasa P, Kim N, Thunders M (2017) Maternal cadmium exposure and impact on fetal gene expression through methylation changes. Food and Chemical Toxicology 109(Pt 1): 714-720.
- WHO (World Health Organization) (2010) Exposure to cadmium: a major public health concern, Public Health and Environment WHO. Geneva, Switzerland.
- Peng L, Huang YT, Zhang F, Chen JY, Huo X (2018) Chronic cadmium exposure aggravates malignant phenotypes of nasopharyngeal carcinoma by activating the Wnt/ beta-catenin signaling pathway via hypermethylation of the casein kinase 1alpha promoter. Cancer Management and Research 11: 81-93.
- Liu T, He W, Yan C Qi Y, Zhang Y (2011) Roles of reactive oxygen species and mitochondria in cadmium-induced injury of liver cells. Toxicology and Industrial Health 27(3): 249-256.
- Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. International Journal of Environmental Research and Public Health 17(11):3782.
- Ahmed AF, Shi M, Liu C, Kang W (2019) Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare) seeds from Egypt and China. Food Science and Human Wellness 8(1): 67- 72.
- Abdul-Ghani AS, Amin R (1997) Effect of aqueous extract of Commiphora opobalsamum on blood pressure and heart rate in rats. Journal of Ethnopharmacology 57(3): 219-222.
- AL-Howiriny T, AL-Sohaibani M, AL-said M, AL-Yahya M, EL-Tahir K, et al. (2004) Hepatoprotective properties of Commiphora opobalsamum ("Balessan"), a traditional medicinal plant of Saudi Arabia. Drugs under Experimental and Clinical Research 30: 213-20.
- EL Rabey HA, AL-Sient AI, AL-Seeni MN, ALSieni MA, ALalawy AI, et al. (2020) The antioxidant and antidiabetic activity of the Arabian balsam tree “Commiphora gileadensis” in hyperlipidaemic male rats. Journal of Taibah University for Science 14(1): 831-841.
- Bouslama L, Kouidhi B, ALqurashi YM, Chaieb K, Papetti A (2019) Virucidal effect of Guggulsterone isolated from Commiphora gileadensis. Planta Medica 85(16): 1225-1232.
- Slinkard K, Singleton VL (1977) Total phenol analysis: Automation and comparison with manual methods. American journal of Enology and Viticulture 28: 49-55.
- Apak R, Guclu K, Özyurek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of Agricultural and Food Chemistry 52(26): 7970-7981.
- Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis 10(3).
- Benzie IF, Strain JJ (1995) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry 239(1): 70-76.
- Brand-Williams W, Cuvelier ME, Berest C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology 28(1): 25-30.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 26(9-10): 1231-1237.
- Lorke D (1983) A new approach to practical acute toxicity testing. Archives of Toxicology 54(4): 275-287.
- Kamel MM, EL Razek A, Ahmed KA, Kamel GM (2011) Exposure of adult male rats to cadmium: Assessment of sexual behaviour, fertility, aggression as well as anxiety like behaviour with special reference to biochemical and pathological alterations. Life Science Journal 8(2): 106-119.
- Tardieu F, Tuberosa R (2010) Dissection and modelling of abiotic stress tolerance in plants. Current Opinion in Plant Biology 13(2): 206-212.
- Hadacek F, Bachmann G, Engelmeier D, Chobot V (2010) Hormesis and a chemical raison d'ětre for secondary plant metabolites. Dose Response 9(1): 79-116.
- Ahmed H, Rashed MM, ALmoiliqy M, Abdalla M, Bashari M, et al. (2023) Antioxidant activity and total phenolic compounds of Commiphora gileadensis extracts obtained by ultrasonic‐assisted extraction, with monitoring antiaging and cytotoxicity activities. Food Science & Nutrition 11(6): 3506-3515.
- ALmulaiky YQ, AL-Farga A (2020) Evaluation of antioxidant enzyme content, phenolic content, and antibacterial activity of Commiphora gileadensis grown in Saudi Arabia. Main Group Chemistry 19(4): 329-343.
- Safhi FA, ALshamrani SM, Jalal AS, EL-Moneim DA, ALyamani AA, et al. (2022) Genetic characterization of some Saudi Arabia’s accessions from Commiphora gileadensis using physio-biochemical parameters, molecular markers, DNA barcoding analysis and relative gene expression. Genes 13(11): 2099.
- AL-Mahbashi HM, EL-Shaibany A, Saad FA (2015) Evaluation of acute toxicity and antimicrobial effects of the bark extract of Bisham (Commiphora gileadensis ). Journal of Chemical and Pharmaceutical Research 7: 810-814.
- Ecobichon DJ (1997) The basis of toxicity testing, CRC press p. 211.
- Nwokocha CR, Owu DU, Nwokocha MI, Ufearo CS, Iwuala MO (2012) Comparative study on the efficacy of Allium sativum (garlic) in reducing some heavy metal accumulation in liver of wistar rats. Food and Chemical Toxicology 50(2): 222-226.
- Tinkov AA, Gritsenko VA, Skalnaya MG, Cherkasov SV, Aaseth J, et al. (2018b) Gut as a target for cadmium toxicity. Environmental Pollution 235: 429-434.
- Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, et al. (2018a) Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environmental Research 162: 240-260.
- Hambach R, Lison DD, Haese P, Weyler J, DE Graef E, et al. (2013) Co-exposure to lead increases the renal response to low levels of cadmium in metallurgy workers. Toxicology Letters 222(2): 233-238.
- Charkiewicz AE, Omeljaniuk WJ, Nowak K, Garley M, Nikliński J (2023) Cadmium toxicity and health effects-A brief summary. Molecules 28(18): 6620-6628.
- Almeer RS, Albasher GI, ALarifi S, Alkahtani S, Ali D, et al. (2019) Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Scientific Reports 9(1): 5825.
- Hamza RZ, AL-Eisa RA, EL-Shenawy NS (2022) Possible ameliorative effects of the royal jelly on hepatotoxicity and oxidative stress induced by molybdenum nanoparticles and/or cadmium chloride in male rats. Biology (Basel) 11(3): 450.
- Chen Y, HU Y, Liu S, Zheng H, WU X, et al. (2016) Whole-body aerosol exposure of cadmium chloride (CdCl2) and tetrabromobisphenol A (TBBPA) induced hepatic changes in CD-1 male mice. Journal of Hazardous Materials 318: 109-116.
- Toppo R, Roy BK, Gora RH, Baxla SL, Kumar PJVW (2015) Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Veterinary World 8(4): 537-540.
- Silveira MAD, Capcha JMC, Sanches TR, De Sousa Moreira R, Garnica MS, et al. (2021) Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis. Scientific Reports 11(1): 5925.
- Promsan S, Jaikumkao K, Pongchaidecha A, Chattipakorn N, Chatsudthipong V, et al. (2016) Pinocembrin attenuates gentamicin-induced nephrotoxicity in rats. Canadian Journal of Physiology and Pharmacology 94(8): 808-818.
© 2025 Amal S Soliman. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






