- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Fibrin Sealants in Neurosurgery: A Structured Literature Review of Efficacy and Complications
Francisco Rivera1*, Matias Ignacio Cosimano2 and Arnau Benet1
1 Department of Neurosurgery, California Neurosurgical Specialists, USA
2 Medical School, University of Buenos Aires, Argentina
*Corresponding author:Francisco Rivera, Department of Neurosurgery, California Neurosurgical Specialists, 2190 Lynn Road, Suite 350, Thousand Oaks, CA 91360, USA
Submission: December 04, 2025;Published: January 06, 2026

ISSN 2637-7748
Volume6 Issue1
Abstract
Objective: Fibrin sealants have become integral in neurosurgical practice, offering both hemostatic
and tissue-sealing benefits across diverse intracranial procedures. Despite widespread use, evidence
regarding their efficacy and safety remains inconsistent. This review aims to synthesize current
applications, outcomes and reported complications in intracranial neurosurgery.
Methods: A narrative literature review with systematic search methods was conducted in accordance
with key PRISMA 2020 principles. PubMed database was searched from inception using combinations
of MeSH terms and keywords related to “fibrin sealant,” “neurosurgery,” “intracranial hemostasis” and
“dural closure.” Eligible studies included Randomized Controlled Trials (RCTs), cohort studies, case
series and reviews reporting clinical outcomes of fibrin sealant use in intracranial procedures. Data were
extracted regarding application type, efficacy and complications. Due to heterogeneity among studies,
results were synthesized narratively.
Results: A total of 22 studies met the inclusion criteria. Fibrin sealants were useful for dural closure,
reduction of Cerebrospinal Fluid (CSF) leakage and hemostasis during tumor and vascular surgeries.
Meta-analyses revealed inconsistent results on preventing postoperative CSF leaks, with some reporting
benefit and others no difference from standard closure. In skull base and vascular procedures, sealants
improved operative visualization and decreased bleeding. Reported complications included postoperative
mass effect, adhesion formation, inflammation and allergic or thromboembolic events.
Conclusion: Fibrin sealants remain valuable adjuncts in neurosurgery, enhancing hemostasis and
facilitating watertight dural closure. However, variability in formulation and expansion properties can
lead to rare but severe complications. Current evidence is based on small series and heterogeneous
studies, underscoring the need for high-quality RCTs to establish guidelines for their safe and effective
use.
Keywords: Cerebrospinal fluid; Complications; Fibrin sealants; Hemostatic agents; Neurosurgery
Abbreviations: CSF: Cerebrospinal Fluid; RCTs: Randomized Controlled Trials; MVD: Microvascular Decompression; DESS: DuraSeal Exact Spine Sealant
Introduction
Intracranial neurosurgery presents remarkable challenges due to the risk of Cerebrospinal Fluid (CSF) leakage, the complex anatomy and the need for hemostasis. Over decades, various hemostatic agents have been developed to address these unique challenges [1]. Among these hemostatic agents, fibrin sealants have emerged as particularly valuable tools in the neurosurgical armamentarium. These biologically derived products, provide effective hemostasis while offering additional benefits as tissue sealants [2]. Fibrin sealants demonstrate remarkable versatility across neurosurgical applications, including: Dural closure, hemostasis during tumor resection and controlling venous bleeding [3]. Despite their widespread adoption and generally favorable safety profile, neurosurgeons must maintain awareness of potential adverse events associated with these products [4]. Unfortunately, current literature provides limited evidence regarding both the efficacy and potential adverse effects of fibrin sealants in intracranial procedures. Fibrin sealants function through a biomimetic mechanism that replicates the final stages of the physiological coagulation cascade. In this process, human fibrinogen is converted to fibrin monomers by thrombin and the resultant fibrin strands are cross-linked by factor XIIIa to form a stable clot that mimics physiological hemostasis [5]. The fibrin matrix formed serves as both a physical barrier to blood loss and a scaffold for cell migration during wound healing. Other specialized variants like integrate the fibrin components into collagen sheets to enhance handling characteristics and facilitate targeted application in neurosurgical procedures [1-3]. Neurosurgeons have adapted these sealants for various applications, including dural closure, embolization procedures, microvascular decompression, transsphenoidal surgery and peripheral nerve repair [2,3]. Among these, the strategic injection of fibrin sealant into cavernous sinus compartments represents a transformative technique in skull base surgery. This technique effectively controls venous oozing from this challenging region and facilitates safer access to deep-seated pathologies minimizing the risk of nerve injury [3]. Moreover, sealants can be used in endovascular embolization for Arteriovenous Malformations (AVM), arteriovenous fistulas and aneurysms [2-6]. However, their use is associated with potential complications, including tissue ischemia, hemorrhage and catheter adhesion [6]. Therefore, this study aimed to investigate the role of fibrin sealants in intracranial neurosurgery, with a particular focus on their efficacy and associated complications.
Methods
Literature search strategy
A narrative literature review with systematic search methods was conducted in accordance with key PRISMA 2020 principles and a PRISMA flow diagram was prepared. The primary objective was to evaluate the role of fibrin sealants and bio adhesives in neurosurgical intracranial procedures. The study selection process is illustrated in the PRISMA flow diagram. A structured search was performed in PubMed up to May 27, 2025. PubMed database was searched from inception using combinations of MeSH terms and keywords related to “fibrin sealant,” “neurosurgery,” “intracranial hemostasis” and “dural closure.”. Boolean operators (AND/OR) were used to refine the search. Additionally, reference lists of relevant articles were screened to identify supplementary studies.
Study selection and eligibility criteria
Studies were included based on predefined inclusion and exclusion criteria. Eligible studies met the following criteria: [1] investigated the use of fibrin sealants in intracranial hemostasis or dural closure, [2] reported clinical or surgical outcomes and [3] were Randomized Controlled Trials (RCTs), cohort studies, reviews or case series. Studies focusing solely on animal models or non-surgical interventions were excluded, as well as non-English publications and studies lacking clear patient outcomes. Screening was performed in two phases: Title and abstract screening, followed by full-text review. After applying the inclusion criteria and quality assessment, 11 studies were considered eligible. An additional 11 studies were identified through backward citation analysis, bringing the total number of included studies to 22. A PRISMA-style flow diagram (Figure 1) summarizes the selection process.
Figure 1:PRISMA 2020 flow diagram illustrating the study selection process.
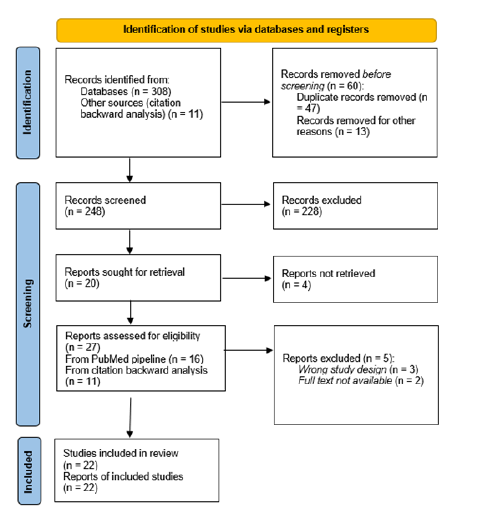
Data extraction and quality assessment
Two independent reviewers (FR, MC) screened articles and extracted data regarding clinical applications, surgical outcomes, efficacy in intracranial hemostasis and reported complications such as sealant expansion, CSF leak, adhesion formation and inflammatory responses. Any discrepancies were resolved through discussion and consensus.
Data synthesis
A narrative synthesis approach was used to summarize the findings from the included studies. Key themes were identified, including patient selection criteria, hemostatic product used, surgical interventions and clinical outcomes. Given the anticipated heterogeneity in study design and outcome reporting, a formal meta-analysis was not performed.
Risk of bias
Since the primary objective of this review was to provide a broad synthesis of the available evidence rather than to critically appraise study quality, a formal risk of bias assessment was not performed. The findings should therefore be interpreted as a narrative synthesis informed by systematic search methods.
Results
We identified 22 articles examining the application of fibrin sealants in intracranial neurosurgery, with particular attention to their efficacy and reported complications (Table 1).
Table 1:Summary of included studies.
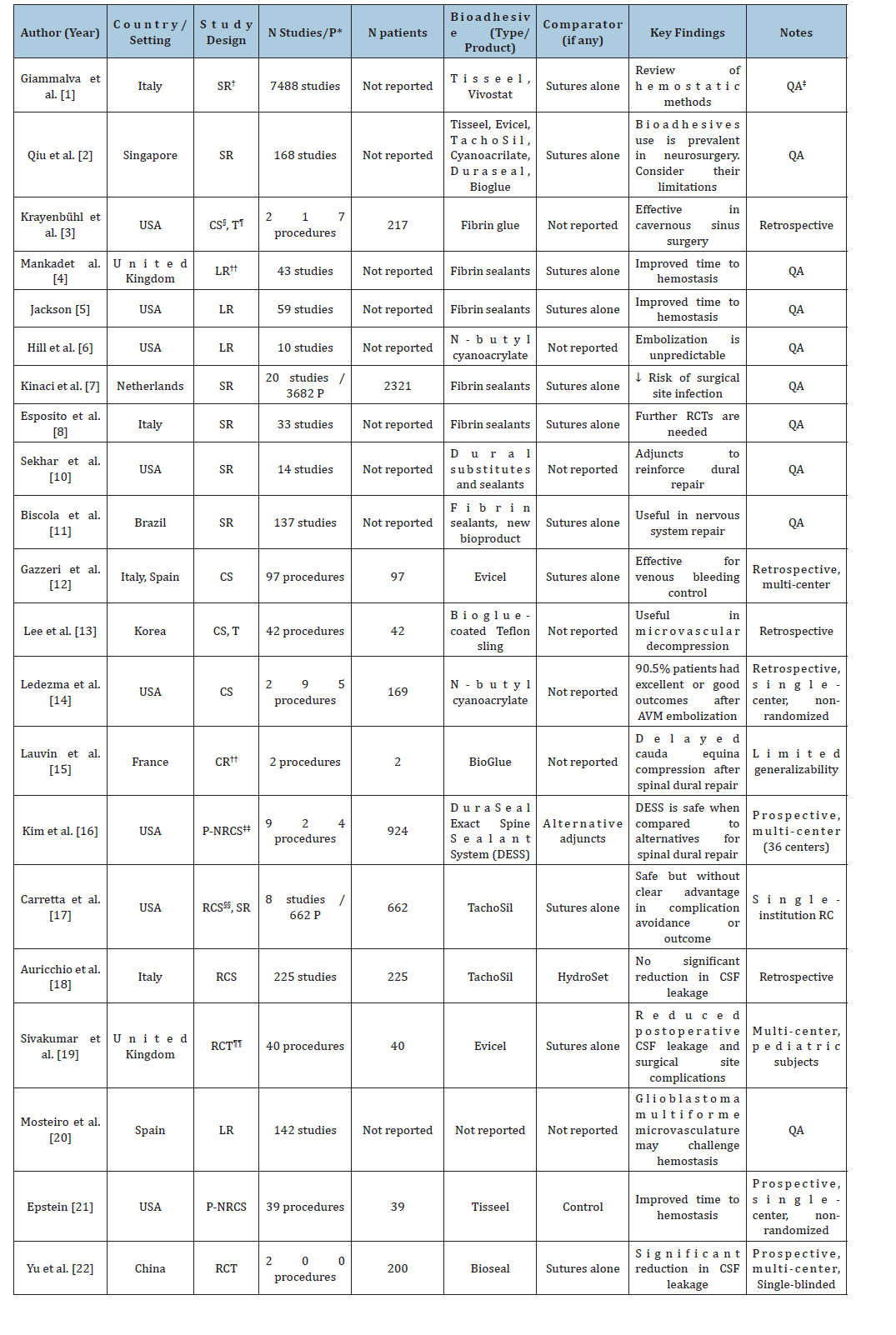
Abbreviations: *P: Patients; † SR: Systematic Review; ‡QA: Qualitative Analysis; §CS: Case Series; ¶ T: Technical Note; **LR: Literature Review; ††CR: Case Report; ‡‡ P-NRCS: Prospective Non-Randomized Clinical Study; §§ RCS: Retrospective Cohort Study; ¶¶RCT: Randomized Controlled Trial
Applications of fibrin sealants in neurosurgery
Dural closure and prevention of cerebrospinal fluid leakage: Sealants have been extensively utilized in neurosurgery to facilitate dural closure and prevent cerebrospinal fluid leakage [7]. A systematic review encompassing 33 studies (2,935 patients) demonstrated that fibrin sealants significantly reduce the incidence of CSF leakage postoperatively [8,9]. However, one meta-analysis of 20 studies involving 3,682 patients found no statistically significant reduction in CSF leakage rates compared to traditional closure methods such as synthetic sealants, collagen or gelatin-based sponges [7]. Fibrin sealants remain a widely adopted adjunct in dural repair, especially in cases of high-risk leaks following tumor resection or neurotrauma [10].
Hemostasis in tumor and vascular neurosurgery: Fibrin sealants can be used as hemostatic agents following the total or partial resection of brain tumors replacing conventional sutures [11]. In a series of 217 cases involving meningiomas, schwannomas, pituitary adenomas, paraclinoid aneurysms and complex basilar aneurysms, fibrin sealants were injected in anatomical “windows” of the cavernous sinus to achieve intraoperative hemostasis. This technique achieved a drier surgical field without any observed clinical complications during postoperative follow-up. Furthermore, postoperative angiographic evaluation demonstrated reestablishment of venous flow within the cavernous sinus within two to three months [3]. In addition, Gazzeri R et al. [12] demonstrated that no patients that were treated with EVICEL to control venous bleeding in cranial procedures needed additional hemostatic procedures [12].
Microvascular decompression in trigeminal neuralgia and hemifacial spasm: A prospective study involving 42 patients undergoing Micro Vascular Decompression (MVD) for hemifacial spasm demonstrated that transposition of the vertebral artery using a fibrin sealant-coated Teflon sling is a safe and effective approach [13]. No symptom recurrence was observed in any cases during a two-year follow-up [13].
Endovascular neurosurgery and embolization procedures: Fibrin sealants are also utilized in endovascular neurosurgery, particularly in the embolization of arteriovenous malformations and other intracranial vascular lesions. A meta-analysis of 295 embolization procedures revealed that fibrin-based adhesives offer superior adhesion properties and biocompatibility compared to other embolic agents. However, those procedures were associated with a higher risk of venous infarction and hemorrhage, especially in complex, high-flow lesions [14].
Adverse events of fibrin sealants: Despite not being a fibrin sealant, DuraSeal has been associated with postoperative volume expansion, a phenomenon that may contribute to parenchymal tissue compression [2,15,16]. This issue prompted the development of a low-swell formulation of DuraSeal. A nonrandomized multicenter study found that the original DuraSeal formulation expanded 38% more than its low-swell counterpart, DuraSeal Exact Spine Sealant (DESS), which exhibited an expansion rate of 19% [16]. Carretta A et al. [17]. in a study that encompassed 662 patients and a systematic review, concluded that the routine use of TachoSil and similar sealants adjunctive to primary duraplasty is generally safe [17]. However, a retrospective review of 225 patients who underwent retrosigmoid craniotomy revealed that TachoSil did not significantly reduce CSF leakage rates [18]. Complications associated with fibrin adhesives include air embolism, cranial nerve compression, infection, systemic allergic reactions and even the formation of de novo aneurysms [15-18]. On the other hand, a multicenter prospective trial in pediatric population undergoing cranial neurosurgical procedures revealed that Evicel (a fibrin sealant) was safe and effective as a primary suture adjunct in this population [19]. Finally, in a systematic review of 28 studies, Esposito et al. [8,9] reported that dural sealants demonstrated no adverse events across the reviewed articles [8,9].
Discussion
This study is, to the best of our knowledge, the first structured literature review to highlight the safety, effectiveness and potential complications associated with the use of fibrin sealants in intracranial neurosurgery. Fibrin sealants are integral to hemostasis in neurosurgical tumor resection, particularly in highly vascularized tumors and structures, where conventional techniques may be insufficient [12] While they are effective in controlling venous bleeding and improving surgical visualization, their use is not devoid of potential adverse effects. Complications such as air embolism and cranial nerve compression have been reported. What is more, glioblastoma multiforme and other highly vascularized tumors create a microenvironment that necessitates the use of fibrin sealants for hemostasis [20]. Beyond cranial surgery, fibrin sealants such as Tisseel have demonstrated benefits in spine surgery, reducing postoperative drain duration and hospital stay [21]. These findings evidence the need for fibrin sealants when seeking hemostasis but also a measured approach to their application, optimizing its hemostatic advantages while mitigating the risk of adverse outcomes. The nuanced application of fibrin sealants in neurosurgery could be re-examined. While their role in dural repair, hemostasis and even vascular procedures is well documented across systematic reviews and case series, the heterogeneity in outcomes and occasional complication profiles caution against uncritical adoption [7,9-14,17,20]. As demonstrated in prior studies, sealant efficacy is not absolute and variability in formulation, expansion properties and tissue interaction can influence outcomes in ways not always predictable [2,8,22]. In this context, this review serves as a reflection of the complexity that underpins clinical decision-making, reinforcing the need for tailored application, formulation-specific vigilance and continued investigation through prospective, controlled studies. There is a critical need for further refinement in bio adhesive technologies to enhance patient safety in neurosurgical practice. While fibrin sealants remain integral to intraoperative hemostasis and dural closure, variability in composition and performance may influence clinical outcomes. Alternative bio adhesives present a potential avenue for improving both efficacy and safety [2,8,9,22]. Sealants such as DuraSeal, although not fibrin-based, have been associated with postoperative volume expansion, a phenomenon that may contribute to parenchymal tissue compression [2,15,16]. This concern led to the development of a low-swell formulation of DuraSeal. Given the reliance on these agents in neurosurgical procedures, rigorous investigation through controlled studies and clinical trials is imperative to establish standardized, evidencebased guidelines for their optimal use.
A recent meta-analysis demonstrated that the use of dural sealants in cranial neurosurgery reduced postoperative CSF leaks and overall infection rate after craniectomy procedures [23]. Therefore, the effectiveness of fibrin sealants is highly dependent on their careful application, appropriate patient selection and a heightened awareness of potential adverse events. Surgeons must balance the advantages of bio adhesives with the inherent risks, particularly when working in confined anatomical spaces. There remains a scarcity in large-scale randomized controlled trials that compare different bio adhesive formulations. Much of the available data is derived from case series and retrospective reviews, underscoring the need for well-designed studies to establish standardized guidelines. Finally, further research is warranted to evaluate the long-term safety, biomechanical properties and potential advantages of emerging alternatives in neurosurgical applications. This study has several limitations. First, the quality and heterogeneity of the included studies may have influenced the findings, as some lacked randomized controlled designs or longterm follow-up. Second, by limiting our search to PubMed, we may have missed studies indexed only in other databases. However, given the substantial overlap, we considered PubMed sufficient for the focused scope of this review. Third, the evidence supporting the use of fibrin sealants in intracranial neurosurgery remains limited, with conflicting data regarding their effectiveness in reducing CSF leakage and achieving hemostasis in complex vascular structures. Finally, complications such as adhesion formation, thromboembolic events and mass effect due to sealant expansion require further investigation, as current reports are largely anecdotal or based on small case series. Future high-quality clinical trials are needed to better define the safety profile and long-term outcomes associated with fibrin sealant use in neurosurgery.
Conclusion
In conclusion, while fibrin sealants play a crucial role in promoting hemostasis and minimizing complications such as CSF leakage and infection, they must be used carefully. Nevertheless, when applied strategically, fibrin-based adhesives can contribute to improved surgical efficiency and better long-term outcomes. Further research is essential to resolve the existing uncertainties surrounding their efficacy, refine their application in complex neurosurgical procedures and ultimately enhance patient safety.
Acknowledgements
Francisco Rivera: Conceptualization; Methodology; Investigation; Writing-original draft; Writing-review & editing. Supervision. Matias Ignacio Cosimano: Methodology; Investigation; Writing-original draft; Writing-review & editing; Visualization. Arnau Benet: Writing-review & editing; Supervision. All authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Conflict of Interest
None.
References
- Roberto GG, Brunasso L, Costanzo R, Paolini S, Umana G, et al. (2021) The role of hemostatic devices in neurosurgery. A systematic review. J Clin Neurosci 89: 151-157.
- Qiu L, Qi See A, Steele TW, Kam King (2020) Bioadhesives in neurosurgery: A review. J Neurosurg 133(6): 1928-1938.
- Krayenbühl N, Hafez A, Hernesniemi JA, Krisht AF (2007) Taming the cavernous sinus: Technique of hemostasis using fibrin glue. Neurosurgery 61(3 Suppl): E52.
- Mankad PS, Codispoti M (2001) The role of fibrin sealants in hemostasis. Am J Surg 182(2 Suppl): 21S-28S.
- Jackson MR (2001) Fibrin sealants in surgical practice: An overview. Am J Surg 182(2 Suppl): 1S-7S.
- Hill H, Chick JF, Hage A, Srinivasa RN (2018) N-butyl cyanoacrylate embolotherapy: Techniques, complications and management. Diagn Interv Radiol 24(2): 98-103.
- Kinaci A, Algra A, Heuts S, O’Donnell D, van der A, et al. (2018) Effectiveness of dural sealants in prevention of cerebrospinal fluid leakage after craniotomy: A systematic review. World Neurosurg 118: 368-376.
- Esposito F, Angileri FF, Kruse P, Cavallo LM, Solari D, et al. (2016) Fibrin sealants in dura sealing: A systematic literature review. PLoS One 11(4): e0151533.
- Esposito F, Angileri FF, Kruse P, Cavallo LM, Solari D, et al. (2017) Correction: Fibrin sealants in dura sealing: A systematic literature review. PLoS One 12(4): e0175619.
- Sekhar LN, Mai JC (2013) Dural repair after craniotomy and the use of dural substitutes and dural sealants. World Neurosurg 79(3-4): 440-442.
- Biscola NP, Cartarozzi LP, Ulian-Benitez S, Barbizan R, Castro MV, et al. (2017) Multiple uses of fibrin sealant for nervous system treatment following injury and disease. J Venom Anim Toxins Incl Trop Dis 23: 13.
- Gazzeri R, Fiore C, Galarza M (2015) Role of EVICEL fibrin sealant to assist hemostasis in cranial and spinal epidural space: A neurosurgical clinical study. Surg Technol Int 26: 364-369.
- Lee SH, Park JS, Ahn YH (2016) Bioglue-coated teflon sling technique in microvascular decompression for hemifacial spasm involving the vertebral artery. J Korean Neurosurg Soc 59(5): 505-511.
- Ledezma CJ, Hoh BL, Carter BS, Pryor JC, Putman CM, et al. (2006) Complications of cerebral arteriovenous malformation embolization: Multivariate analysis of predictive factors. Neurosurgery 58(4): 602-611.
- Lauvin MA, Zemmoura I, Cazals X, Cottier JP (2015) Delayed cauda equina compression after spinal dura repair with bioglue: Magnetic resonance imaging and computed tomography aspects of two cases of “glue-oma.” Spine J 15(1): e5-e8.
- Kim KD, Ramanathan D, Highsmith J, Lavelle W, Gerszten P, et al. (2019) Duraseal exact is a safe adjunctive treatment for durotomy in spine: Postapproval study. Global Spine J 9(3): 272-278.
- Carretta A, Epskamp M, Ledermann L, Staartjes VE, Neidert MC, et al. (2022) Collagen-bound fibrin sealant (TachoSil®) for dural closure in cranial surgery: Single-centre comparative cohort study and systematic review of the literature. Neurosurg Rev 45(6): 3779-3788.
- Auricchio AM, Martinelli R, Offi M, Nichelatti M, Valeri F, et al. (2025) Dural and cranial reconstruction techniques in retrosigmoid craniotomy: Key factors associated with CSF leaks in 225 patients. Neurosurg Focus 58(2): E8.
- Sivakumar G, Magdum S, Aquilina K, Kandasamy J, Josan V, et al. (2024) Safety and effectiveness of Evicel® fibrin sealant as an adjunct to sutured dural repair in children undergoing cranial neurosurgery. Childs Nerv Syst 40(9): 2735-2745.
- Mosteiro A, Pedrosa L, Ferrés A, Diao D, Sierra À, et al. (2022) The vascular microenvironment in glioblastoma: A comprehensive review. Biomedicines 10(6): 1285.
- Epstein NE (2014) Tisseel utilized as hemostatic in spine surgery impacts time to drain removal and length of stay. Surg Neurol Int 5(Suppl 7): S354-S361.
- Yu R, Zhu W, Kocharian R, Ilie B, Wang Z, et al. (2022) A multicenter, prospective, randomized clinical study to evaluate the efficacy and safety of fibrin sealant as an adjunct to sutured dural repair. Chin Med J 135(20): 2506-2508.
- Aguiar CC, Filho U, Bonatti BF, Oliveira MD, Ely V (2025) Comparative effectiveness of dural sealants versus conventional dural closure methods in cranial neurosurgery: A systematic review and meta-analysis. Neurosurg Rev 49(1): 33.
© 2025 Francisco Rivera. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






