- Submissions

Full Text
Significances of Bioengineering & Biosciences
Sterilization Validation and Routine Control: Insight from ISO TC 198 Documents
Hideharu Shintani*1 and Katsumi Nakamura2
1Faculty of Science and Engineering, Chuo University, Japan
2Raven Japan Co Ltd, Japan
*Corresponding author:Hideharu Shintani, Faculty of Science and Engineering, Chuo University, 1-13-27, Kasuga, Bunkyo, Tokyo 112-8551, Japan
Submission: September 10, 2025; Published: September 30, 2025

ISSN 2637-8078Volume7 Issue 5
Abstract
Sterilization, disinfection and decontamination methods are widely used to inactivate microorganisms, some of which can be harmful to humans. Sterilization validation is regulated globally by the International Organization for Standardization (ISO), particularly through Technical Committee (TC) 198, which addresses sterilization validation and routine control. These processes are crucial for ensuring the scientific and rational inactivation of microorganisms contributing to safer practices worldwide. Typically, Biological Indicators (BIs) are used for sterilization efficiency assessment. This paper will discuss sterilization validation, routine control and key considerations for their implementation in detail.
Keywords: Sterilization; Disinfection; Validation; Routine control; Biological indicator; International organization for standardization; Bioburden; D value
Introduction
To inactivate microorganisms, several approaches are widely used [1-19] including ethylene oxide, moist heat, dry heat, gamma-rays, formaldehyde gas, hydrogen peroxide gas and plasma sterilization [2-19]. Most of these methods are discussed in ISO TC 198 [1]. However, there are still technical concerns regarding sterilization validation and routine control. In this review we will discuss these points concretely in depth.
Bioburden
Bioburden in the environment can be classified into three categories: Airborne microorganisms, falling microorganisms and adherent microorganisms. The detection of airborne microorganisms may vary depending on the type of air sampling equipment used [20]. Representative airborne and falling microorganisms typically include Micrococcus spp., Staphylococcus spp., Bacillus spp. and Corynebacterium spp. [20]. The number of falling microorganisms is approximately one-tenth that of airborne microorganisms, indicating that the majority of bioburden exists in the air. Among airborne and falling microorganisms, Micrococcus spp. is the most predominant [20]. Bioburden typically comprises microorganisms that are damaged by factors such as UV irradiation, environmental stress or nutrient starvation; therefore, they are not always in a healthy state [21]. Consequently, sterilization validation and routine control should be performed using microorganisms that have survived exposure to various sterilant and stresses [21].
Constituents of the recovery medium for bioburden
Generally, 1% peptone solution containing 0.1% Tween 80 adjusted to pH7.2 is recommended to recover bioburden. However, when the recovery rate of Micrococcus spp., Staphylococcus spp., of Corynebacterium spp. is low, the composition of the recovery medium should be validated. This is because chloride has an inhibitory effect on damaged microorganisms. Therefore, a phosphate buffer at pH7.2 with the same composition. The addition of Tween 80 is necessary due to the hydrophobic nature of the spore outer layer of Bacillus spp., which are among the most resistant microorganisms to the sterilization procedures. For this reason, Biological Indicators (BIs) are commonly used [22]. Sots of bioburden may differ depending on the environment of the user’s facility, therefore validation study of recovery medium constituents must be conducted by the users themselves. The reason bioburden differs depending on the facility is because employees differ, which means sorts of humans differ. The type of bioburden may vary depending on the environment of a given facility, therefore validation studies of recovery medium constituents must be conducted by individual users. Differences in bioburden across facilities are largely attributable to variations in employees, as human-associated microorganisms differ accordingly.
Information on bioburden recovery medium
In most cases, bacterial spores are the primary targets to inactivate as they are more resistant to sterilants than vegetative cells. To cultivate spores, peptone solution containing Tween 80 (polysorbate) is frequently used. Examples of recovery medium constituents are provided in ISO 11737-1 [23]. As a bioburden, the number of vegetative cells generally exceeds that of spores [20]. In sterilization validation and routine control, it is necessary to determine only the numbers and types of microbial species present [24-26]. Determination of resistance (decimal reduction value, D value) is not always required in ISO 11138 series [22, 24-28] and depends on the sterilization procedure under consideration, which will be discussed later. Generally, assessing bioburden resistance in sterilization validation and routine control is not required except in the case of absolute bioburden procedures as described later.
Selection of recovery method for bioburden [23]
When medical devices can be placed in a stomacher bag, the stomacher recovery method is recommended as it is generally a higher recovery rate. However, bioburden may be damaged during the recovery process: Therefore, the addition of injuryrecovery agents to the recovery medium is necessary [21,29]. The recovery rate of the cleaning method is lower than that of the stomacher recovery method, while the efficacy of the culture medium immersion method may vary depending on the size of the medical devices [23]. ISO 11137 describes of SIP (Sample Item Portion) in which only part of a medical device is sampled [30]. However, SIP is not always recommended because distribution of bioburden follows a Poisson rather than a normal distribution [20]. This indicates that the sampled portion may not contain any microorganisms, potentially leading to inaccurate results. Therefore, the SIP approach in ISO 11137 requires reconsideration. There is a procedure to remove bioburden from the materials using ultrasonic treatment: This procedure should be considered: However, bioburden may be damaged during the recovery process. Sterilization validation is the task of detecting the presence of bioburden remaining after exposure to heating, chemicals, radiation, UV irradiation and so on, with the recover targets being damaged microorganisms [21]. By contrast, commercially available culture media are designed for healthy microorganisms. Notably, there is a controversy regarding the nutritional requirements of healthy versus damaged microorganisms. The latter requires eutrophic conditions [21,29].
In the case of recovering healthy microorganisms, there is no significant difference in cultivation efficiency among the commercially available Soybean Casein Digest Agar (SCDA) culture media (Table 1). However, damaged microorganisms may be influenced by differences in SCDA composition (Table 2). For example, if calcium with or without magnesium is removed from SCDA medium with EDTA, healthy microorganisms can still survive by utilizing the calcium and magnesium contained within their cells. However, damaged microorganisms cannot survive due to the absence of these metals within their cells [12,21,29,31]. For healthy microorganisms, growth does not differ depending on the types of SCDA supplied from the culture medium manufacturer [Table 1] [21]. The approval range of recovery is from -50% to +300% of the labelled population, indicating that all manufactures are within acceptance limits [21,22]. In case of damaged spores, growth differs significantly depending on the SCDA culture medium supplier (Table 2). This difference is more evident as the extent of damage increases, for example, compare 3 minute and 5 minutes exposure in Table 2. When 0.5% glucose was added to the medium (Table 3), recovery of the population improved compared with Table 2, for both 3 minute and 5-minute heat exposure.
Table 1:Initial population of the Biological Indicator (BI) using Geobacillus stearothermophilus ATCC 7953 for moist heat sterilization.
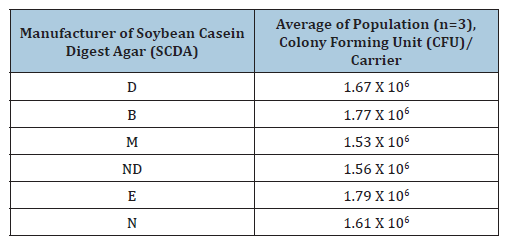
Table 2:Injured population of BI using Geobacillus stearothermophilus ATCC 7953 exposed with moist heat sterilization at 121 oC for 3 minutes or 5 minutes.
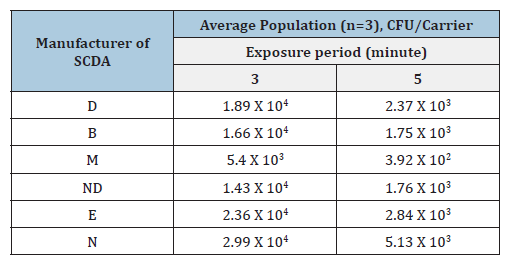
Table 3:Injured population of BI using Geobacillus stearothermophilus ATCC 7953 exposed with moist heat sterilization at 121 oC for 3 minutes or 5 minutes, respectively and cultivated in SCDA containing 0.5% glucose.
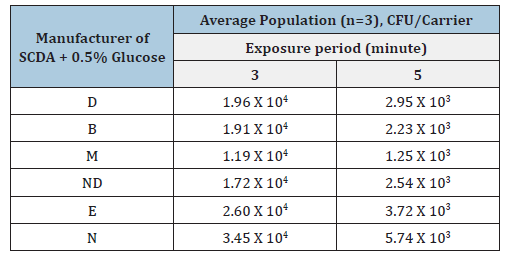
Table 4:Effect of chemicals added on the microbial recovery in the SCDA culture medium supplied from M company BI using Geobacillus stearothermophilus ATCC 7953 treated with moist heat sterilization at 121 oC for 4 minutes.
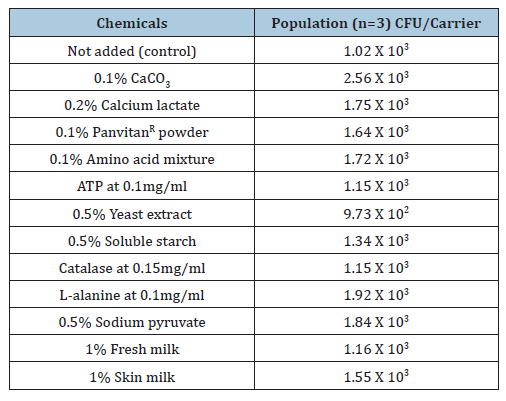
All recovery were within acceptance limits (-50% to +300%). In the place of 0.5% glucose several other chemicals were added to the SCDA culture medium supplied by company M and the spore was damaged at 121 oC for 4 minutes with moist heat sterilization (Table 4) [21]. The control is medium without supplementation. Compared with the control, almost all chemicals except 0.5% yeast extract (within experimental error) are useful, especially calcium addition. Data from Table 4 indicate calcium, L-alanine, amino acid mixture, sodium pyruvate and glucose improve recovery of damaged spores reducing differences between media from different suppliers [21]. The main component in commercially available amino acid mixtures is L-alanine, which promotes recovery from injury. For damaged microorganisms, lower cultivation temperature combined with a longer incubation period is necessary as they exhibit an extended lag phase. Depending on sterilization procedures, the type of sort of microorganisms, sterilization period and the chemicals used for damage recovery may differ; therefore, these factors must be validated by the users. The reasons for these differences include variation in the mechanism of sterilization, the site and degree of microbial damage and nutritional requirements. Based on our experimental findings, the addition of amino acid mixtures, glucose and calcium is useful for promotion recovery from injury [21]. Glucose concentration higher than 0.5% may be even more effective, although this has not been experimentally confirmed and remains speculative.
Bioburden as oligotrophic microorganisms
Waterborne microorganisms such as Pseudomonas spp. and Legionella spp. can adapt to nutrient-poor environments; therefore, they may thrive under malnourished conditions. The Association for the Advancement of Medical Instrumentation (AAMI) and ISO recommend the use of R2A culture medium for oligotrophic microorganisms. Culture conditions for healthy oligotrophic microorganisms typically involve lower temperatures and longer incubation periods. For damaged oligotrophic microorganisms, initial cultivation under enriched nutrient conditions at lower temperature for longer periods may be necessary to allow recovery, after which they can be transferred back to nutrient-poor conditions. Oligotrophic microorganisms are of concern because of their association with biofilm formation and in the case of gramnegative species endotoxin production. Endotoxins are related into the environment after the death of gram-negative microorganisms, creating a risk when reused medical devices contaminated with endotoxins come into direct contact with body fluids. Inactivation of endotoxin is addressed in Good Manufacturing Practice (GMP) guideline and in ISO 13485 [32]. To prevent biofilm formation, dead legs in fluid pathways must be avoided. However, disinfection with high-temperature water has proven insufficient to eliminate endotoxins. The role of endotoxins will be discussed in the dry heat sterilization section later.
Parametric Release by Ethylene Oxide Gas (EOG) Sterilization and Dosimetry Release by Gamma- Ray Sterilization
If all physical parameters are within the approved acceptance limits, sterilized items can be shipped without performing BI cultivation or sterility testing [33]. In case, only the physical parameters are required. In ISO 11135 includes a description of parametric release [33]. Although ISO 11135 permits parametric release, aeration must still be conducted after EOG sterilization to reduce residual EOG to within the approved limit (e.g., 25ppm) [34]. Therefore, parametric release in EOG sterilization is not always practical. In practice sterility testing may be performed during the 2-week aeration period while residual EOG levels are reduced to acceptable limits [34]. Several reasons make EOG unsuitable for parametric release. Accurate, reproducible and long-term reliable humidity sensors for EOG chambers are unavailable because EOG polymerization lead to deposit on the sensor. Real-time online determination of EOG concentration in the chamber is possible only by Infrared Ray (IR) spectroscopy. Gas-Liquid Chromatography (GLC), although more sensitive than IR, is not suitable for realtime monitoring because of time requirements. Furthermore, due to variation in EOG concentration within chamber, measurements would need to be taken at multiple locations; however, this is practically impossible to implement. The half-cycle procedure is not approved for parametric release.
Instead, sterilization time must be determined based on the D-value obtained at the most-difficult-to sterilize location in an actual EOG sterilizer. A Sterilization Assurance Level (SAL) of 10-6 must be achieved for final sterilization. In contrast, conducting sterility testing at an SAL of 10-2 is both redundant and contradictory, even though sterility testing is not required for parametric release in EOG sterilization [33]. Parametric release is both useful and practical; however, it should be discussed within ISO TC 198, Working Group (WG) 3 on moist heat sterilization [35]. In Japan, parametric release for moist heat sterilization is already approved for new drug applications [36,37]. Parametric release is a concept applied in routine control rather than in validation studies. In contrast, a validation study evaluates the relationship between BI results and the physical parameters used in parametric release. ISO 11135 specifies the required number of physical sensors and BIs [33]. However, this specification is based solely on chamber volume. Even when chamber volume is identical, cold spot (the most difficult locations to sterilize) may vary depending on loading patterns. Thus, chamber volume alone cannot determine the required number of BIs and physical sensors used. Therefore, ISO 11135 requires revision. BI sensor and installation sites must include validated cold spots.
In routine control, BIs and physical sensors should be concentrated at cold spots. When all BIs show no growth after exposure, a SAL of 10-6 can be considered achieved. Loading patterns in validation studies and routine control must be identical to prevent changes in cold spot locations. If identical patterns are difficult to achieve, dummy or defective products may be used to replicate loading conditions. To ensure sterility assurance, the equivalence between real and dummy or defective products must be validated. In parametric release of moist heat sterilization, in addition to parameters such as temperature, pressure, time. D value, Z value, L value, F value and Fo value must also be determined [38]. It is essential to recognize that sensors must be installed at cold spots. Fo values calculated at hot spots (easily sterilized locations) are meaningless. The calculation method for these values is described previously [38]. Dosimetry release by gammaray sterilization can be performed based on accurate determination of absorbed doses [30]. Unlike parametric release, which requires a validated relationship between BI results and physical sensors, dosimetry release requires only accurate dose measurement. However, sterility restings are still frequently required under ISO 11137 [30]. According to this ISO 11137, the coefficient of variation for dosimeters must be <2%. Detailed procedures for determining absorbed doses are provided in ISO 11137 [30].
Moist Heat Sterilization [35]
You should accurately determine several physical factors such as temperature, pressure and time. It is necessary to validate that the relationship between physical parameters and the death of BIs installed in cold spots is satisfied. In validation studies it is necessary to identify cold spots accurately and reproducibly and in these cold spots BIs must be installed close to physical sensors. In the case of parametric release, temperature in cold spots must be useful to determine the Fo value. It is essential for sterilization validation to obtain the Fo value from physical sensors installed in cold spots. Under sterilization conditions, loading patterns identified in the validation study must be reproduced in routine control. If this is done consistently, sterility assurance in routine control will be successful. Routine control must be carried out in correlation with the validation study. The resistance of BIs (D value) may differ depending on the carrier materials and/or primary packaging materials of the BIs [12]. As mentioned earlier and in citations [12,29], D values may also differ depending on the constituents of the culture medium. To obtain a reproducible sterility assurance, BIs may not always be a versatile tool for achieving reproducible results. However, the death of BI installed in cold spots indicates the death of all bioburdens. This is because the D value and the population of BIs is much larger than that of bioburdens.
Chemical Indicators (CIs) are not recommended for sterilization validation. Class 1 CIs change color at a certain temperature, but no further change can be observed even if the temperature continues to increase [39]. This means they lack the information needed to confirm maintenance of a certain temperature. In contrast, cumulative CIs such as Class 5 and 6 CIs change color when a certain temperature is maintained over time, indicating that they have a similar function to Fo [38,39]. In validation studies if the relationship between BIs and cumulative CIs is satisfactory, cumulative CIs may be useful for validation studies and/or routine control [39]. ISO TC 198 WG 8, CI, must work on the use of cumulative CIs for validation studies in future ISO documents. A Process Challenge Device (PCD) is a device used to recreate the resistance of BIs installed in cold spots as described in ISO 14161 [24]. PCDs are used in routine control and they are not installed in cold spots; instead, they are placed in hot spots (easy-to-sterilize locations) in the sterilization chamber. Never place PCDs in cold spots during routine control as excessive exposure may occur and damage the quality of health care products. In validation studies the resistance of PCD and that of BIs installed in cold spots should show a satisfactory relationship. The determination of sterilization period is described in ISO 14161 and includes the following procedures: Overkill procedure, Combined BI and bioburden procedure, absolute bioburden procedure and half-cycle procedure [24,26].
In practice for moist heat sterilization at 121.1 oC with a 12D exposure the overkill procedure is normally conducted. The D-value using a practical sterilizer is almost three times that of the labelled D value of the BIs, corresponding to 4.5 to 7.5 minutes [27,32]. If the products are not tolerable to the 12D exposure such as rubber products, it is necessary to consider alternative sterilization procedure such as Ethylene Oxide Gas (EOG) or gamma-ray irradiation sterilization. These sterilization procedures will be discussed below. Unlike EOG sterilization, the moist heat method does not necessarily require consideration of coexistence.
Overkill procedure
The initial population of BI spore should be 106 Colony- Forming Units (cfu)/carrier and the SAL of 10-n (n=6 when medical devices directly contact body fluid, otherwise n=3), which defines the overkill procedure. For validation, the former requires 12D exposure and the latter a 9D times exposure. The D value should be calculated using the BIs (in case of moist heat sterilization, Geobacillus stearothermophilus (G. stearothermophilus) ATCC 7953 [27], the spore former the most resistant to moist heat sterilization) placed in the cold spots of the practical sterilizer and/or in cold spots within products inside the sterilizer. The D value printed on the BI label should not be used for validation and/or routine control as it intended solely for quality assurance by the BI manufacturer [22]. The labelled D value is obtained using a Biological Indicator Evaluator Resistometer (BIER), which has a very small chamber volume and differs from a practical sterilizer [2,24]. Therefore, the D value for sterilization validation should be determined using BIs placed in the cold spots of the practical sterilizer. The procedure for D value determination using a practical sterilizer is as follows: BIs are placed at several portions within the loaded sterilizer and exposed for a time sufficient to obtain fraction-negative survivors (spore log reduction time, SLR, of 5~10-2 portion).The D value is then calculated using fraction-negative method such as the Spearman-Karber Method or the Stumbo-Murphy-Cochran Method as described in ISO 14161 [24]. Fraction-negative times correspond exposures where some BIs survive and others are inactivated i.e., SLR of 5~10-2.
After sterilization if surviving BIs are detected, these locations are considered cold spots often near drain or ceilings. Cold spots may occur in single or multiple locations. If the material of the products is heat-resistant, the overkill procedure may be used for moist heat sterilization, as it remains within the safety zone provided the material tolerates the process. Notably, both GMP quality assurance and sterilization validation requirements must be satisfied. This is because the ISO 9000 series officially cite all documents of ISO TC 198 documents [25]. ISO 14161 was revised to ISO 11138-7 [26].
Combined BI and bioburden procedure
When performing this procedure in moist heat sterilization, it is necessary to determine the number of bioburdens. The initial population of BI used for the sterilization procedure must be the average bioburden +3 Standard Deviations (SD). In this context, BI should be self-made. Using the prepared BI, the sterilization period can be validated. According to ISO 14161 and ISO 11138-7 [24-26], commercially available BI with a population of >103 cfu/carrier can be used in place of self-made BI. Notably, self-made BI with a population of average bioburden +3SD is also acceptable. Regarding self-made BI, you must follow the requirements of ISO 11138-1 and ISO 11138-3 as you are regarded as the BI manufacturer [27]. ISO 11138-1 and ISO 11138-3 are ISO documents required for BI manufacturers. Therefore, it may be more appropriate to use commercially available BI with a population of 103 cfu/carrier after evaluating the bioburden at your facility.
Absolute bioburden procedure
It is necessary to determine the resistance of the bioburden; the most resistant bioburden should be utilized in place of commercially available BI. For the initial population it should be the average bioburden +3SD, for which BI is self-made. Using this selfmade BI, sterilization validation must be performed to attain SAL of 10-6. Self-made BI must comply with the requirement of ISO 11138- 1 and that of ISO 11138-3. This procedure is mostly applied to facilities produced medical devices and medicines that are sensitive to heat. Generally, it is not required to determine the resistance of the bioburden, but in the absolute bioburden procedure, the resistance must be determined by using BIER according to the requirement of ISO 11138-1, ISO 11138-3 and ISO 11138-7. These ISO documents are intended for the BI manufacturers and BIER requirement is a major obstacle to applying the absolute bioburden procedure world-wide. In ISO 11135 for EOG sterilization [33], the requirements for the absolute bioburden procedure differ from those in ISO 14161 and ISO 11138-7 as discussed later in the EOG section.
Half-cycle procedure
This procedure doubles the time from the initial population of 106 cfu/carrier to an SLR of 5 to 10-2, indicating exposure to 12 D-16D in ISO 11135 [33] for EOG sterilization. There is no description of the half-cycle procedure in moist heat sterilization [35]. This is because few products can withstand heating for a half-cycle procedure. Quality assurance requirements under GMP may not be satisfied using the half-cycle procedure. Only the EOG sterilization procedure is applicable for long exposures. A report compared the degree of degradation after exposure to moist heating, gamma-rays and EOG [40]. According to this report, the order of degradation degree is gamma-ray, moist heating followed by EOG. Typically, most of materials used for health care products follow this order [40]. Detailed are described in Annex A in ISO 11137 [30].
EOG Sterilization [33]
The mechanism of EOG sterilization is the alkylation of nucleobases such as DNA and RNA, protein, lipid, carbohydrate and other biomolecules with HOCH2 CH2 O-derived from EOG [11,41-43]. The D value decreases at higher temperatures and EOG sterilization efficiency improves at higher temperatures. However, EOG polymerization may easily occur at higher temperatures. The general temperature for EOG sterilization is 50 °C - 60 °C. Below 50 °C, it may be difficult to maintain vaporization of EOG within the sterilizer. Distribution of temperature in the equipment qualification test, using an empty chamber of the EOG sterilizer and in the operational qualification test in the loaded sterilizer when injecting EOG into the chamber, must both be validated to demonstrate a satisfactory relationship, which may be difficult due to the possibility of EOG adsorption to the products within the chamber. In the operational qualification test, the number of physical sensors differs depending on the volume of preconditioning chamber in ISO 11135. For chambers of <2.5m3, five temperature sensors and one humidity sensor are required. For chambers greater than 2.5m3 two additional temperature sensors and one additional humidity sensor must be added for each 2.5m3 increase. When loading products into the chamber, it is necessary to validate and confirm that the distribution of temperature and humidity is uniform throughout the chamber. The concentration of EOG for sterilization is typically 500-800mg/L [33]. If it is <300mg/L, a prolonged sterilization period is required. If it exceeds 1200mg/L, sterilization efficiency does not increase further. Up to 1200mg/L, sterilization efficiency approximately doubles when the concentration is doubled.
However, high concentration and high pressure may cause condensation of water and EOG within the sterilizer chamber. To avoid condensation, it is necessary to maintain a high chamber temperature, but EOG polymerization may occur at higher temperatures. Therefore, the use of 500-800mg/L of EOG concentration is considered appropriate with scientific rationale [33]. The Relative Humidity (RH) is appropriate at 60% [41]. As a humidity sensor, the saturated salt method is described in JIS Z-8806 [42]. In the EOG sterilization process, low pressure and vacuum are applied followed by subsequent injection of steam and then EOG. Notably, simultaneous introduction of steam and EOG may decrease sterilization efficiency and therefore must be avoided. EOG sterilization efficiency at humidity level below 30% is considered invalid because sterilization efficiency is almost null. Water is essential for EOG sterilization due to its role as a carrier of EOG to DNA and RNA in BI spores. It is challenging to determine RH accurately in the presence of EOG and under pressure because EOG polymerized deposits may interfere with the accuracy of the humidity sensor. The greatest bottleneck of EOG sterilization depends on the development of humidity sensors that can supply accurate and reliable humidity readings. The color of EOG polymerized deposits ranges from white to brown depending on their molecular weight.
After EOG sterilization it is necessary to conduct aeration to remove EOG residues and reactants such as Ethylene Chlorohydrin (ECH), Ethylene Glycol (EG) and ethyl cellosolve. Aeration is commonly performed at 60 °C and the higher the temperature, the greater the aeration efficiency. Increasing the number of aeration cycle may shorten the total aeration time. The aeration period may differ depending on the characteristics of the products such as polarity and/or hardness. Cellulose and Polyvinyl Chloride (PVC) which have higher polarity may require longer aeration periods, whereas polyethylene or polyester with relatively lower polarity may require shorter period. In the case of PVC the presence of plasticizer, Di 2-Ethylhexyl Phthalate (DEHP), which comprises about 50% of PVC is problematic. It is explained that EOG is absorbed not by PVC itself, but by DEHP within PVC. The aeration period for cellulose or PVC requires at least two weeks at room temperature. Sterility tests can be performed during the aeration period; therefore, as I mentioned earlier, parametric release is not practical even though it is described in ISO 11135 [33,41]. Aeration periods for soft materials such as natural rubber or soft plastic polymers are short, whereas those for hard materials such as polyacrylate polymers or polystyrene resins are long. Based on Fick’s law of diffusion within materials, softer materials allow EOG to diffuse quickly and leave lower amounts of residue.
Water extraction of EOG-sterilized medical devices and GC analysis are described in ISO 10993-7 [34], however, water extraction yields much lower amounts than ethanol extraction [11,43], indicating that the description in ISO 10993-7 should be revised. Extraction with organic solvents such as ethanol or acetone yields much higher amounts than water extraction [11,43]. In the case of ethanol extraction, there is a possibility of forming ethyl cellosolve; therefore, acetone rather than ethanol is a more appropriate organic solvent for EOG extraction. The residual limits of EOG, ECH and EG are <25ppm, 25ppm and 250ppm, respectively [34]. In ISO 11135, specific examples of the numbers of physical sensors and BIs required are described based on chamber volume. The number of BI portions is 20 for <5m3, for 5-10m3, 2 portions are added for every additional 1m3 and for 10m3, 2 portions are added for every additional 2m3. The number of physical sensors is 10 for <5m3, for >5m3, 1 is added for every additional 1m3 and for >10m3, 20 sensors may be set.
Estimation procedures to calculate sterilization period of EOG sterilization
As described earlier for moist heat sterilization, the procedures include the half-cycle procedure, absolute bioburden procedure, combined BI and bioburden procedure and overkill procedure [24- 26]. Most of these have been described previously except for the modified absolute bioburden procedure, which is described only in ISO 11135 [33]. If you determine the resistance of bioburden by BIER, the D value should be that of the most resistance bioburden and the initial population should be the average population +3SD with an SAL of 10-6 required. Typically, 7D to 8D exposures are required. If you do not determine the resistance of bioburdens and the calculated number of bioburdens is fewer than 100cfu, you can use a D value to 1.5 to 2 times greater than that of the labelled BI and the rest is the same as mentioned above. If you do not determine the resistance of bioburdens and the number of bioburdens is >100cfu, you can calculate the D value using the fraction negative procedure with the rest being the same as mentioned above. The D value estimation procedures using the fraction negative method are as follows [24-26]; the Spearman-Karber Procedure (SKP) and the Stumbo-Murphy-Cochran Procedure (SMCP). Detailed calculation procedures are available in ISO 11138-7 [26].
A comparison of D-value accuracy is available in citation [44]. Concerning D-value calculation using the survival curve method (EN) the procedure is as follows: The logarithm population of the BI is plotted on the y-axis and the exposure time on the x-axis. A total of five exposures including one unexposed control are used with four BIs per exposure (a total of 20 BI sheets). A straight line is then drawn using the least-squares method., called the survivor curve. The correlation coefficient is required to be >0.8 [22,28]. If a shoulder phenomenon is present between the initial population (N0) and 0.5 N0, it must be neglected [22,28]. The BI used for EOG sterilization is described in ISO 11138-2, which is Bacillus atrophaeus (B. atrophaeus) ATCC 9372 [28].
Gamma-Ray Sterilization [30]
The mechanism of gamma-ray sterilization involves injury to nucleobases such as DNA and RNA caused by OH radicals generated during irradiation. The followings points are the important points to consider whether the material can withstand sterilization and whether the bioburden will be effectively killed by the absorbed doses. Sterilization absorption doses are defined in ISO 11137 and citations [45,46], which include ISO 11137 Method 1, ISO 11137 Method 2, Verification Dose (VD) max 25kGy, and VD max 15kGy. In ISO 11137 Method 1, the verification dose to achieve an SAL of 10-2 is applied to 100 products followed by sterility testing. Survivors may represent seriously damaged bioburdens, thus if validation studies of the culture medium or culture temperature are inadequate, sterility testing may fail or underestimate these damaged bioburdens (Tables 2-4 and citation [21]). In radiation sterilization, OH radicals are essential: Therefore, the presence of OH radical scavengers such as starch, charcoal or serum albumin may interfere with the correct estimation of damaged bioburden. Failure to recover damaged bioburdens may compromise sterility assurance in gamma-ray sterilization validation. Table 1 in ISO 11137 Method 1 [30,45] describes the determination of bioburden resistance using SCDA culture medium and enhanced resistance. Although sterilization doses may fall within a safe range, they must be reconsidered to balance sterility assurance with material and functional compatibility as highlighted in ISO 11737-1 [23].
Recovery procedures in ISO 11737-1 estimate recovery rates and correction factors for inoculated spores on products. The composition of the recovery medium may differ depending on material type, sites and microorganisms; therefore, recovery methods should be validated by users themselves. In gamma-ray sterilization, it is important to achieve both a specific SAL and material/ functional compatibility [30,45]. For medical device applications to authorities in Japan, it is recommended to sterilize with twice the irradiation dose (three-to four-times in the case of electron beam sterilization [46]) and to perform safety tests immediately after sterilization and again six months postexposure in accordance with ISO 11137 A.2 with data submitted to the authorities [47]. Sensitive materials prone to degradation during gamma-ray sterilization include Polypropylene (PP), PVC and TeflonR (Annex A of ISO 11137) [30]. Polymer color may turn yellowish upon irradiation due to phenol-type antioxidants such as Butylated Hydroxy Toluene (BHT). Replacing BHT with phenoltype antioxidants or hindered amines (heterocyclic compounds) can significantly reduce yellowish. Aromatic polymers such as polystyrene are more resistant to gamma-ray sterilization than aliphatic polymers such as PP as the pi electrons in benzene rings help stabilize OH radicals. It is necessary to consider not only the polymer itself but also antioxidants (e.g., BHT) or plasticizers (e.g., DEHP) when evaluating degradation [30].
Degradation or crosslinking of polymer materials upon gamma-ray irradiation is classified by activation energy at 20kcal/ mol. However, no polymer exhibits exclusively crosslinking or degradation. All polymers present both degradation and crosslinking, with the latter requiring higher activation energy than degradation. If the difference between crosslinking and degradation activation energies is <20kcal/mol, the polymer is classified as crosslinking type polymer; if >20kcal/mol, it is classified as degradation type [30,45]. Stability tests include tensile testing, compressive strength testing, elution testing and breaking strength testing [4,30]. Polystyrene (PS) is considered resistant to gamma-ray irradiation; however, styrene oligomers (monomer to trimer) known as endocrine disruptors, have been confirmed to leach from PS upon irradiation [48]. Similarly, bisphenol A, also an endocrine disruptor, has been reported to leach from irradiated Polycarbonate (PC) [48]. Since even trace amounts of styrene oligomers and bisphenol-A are harmful effect to humans, the development of polysulfone and PC materials resistant to gammaray sterilization is desirable.
Dry Heat Sterilization [49]
The sterilization condition for dry heat is defined at Water Activity (Aw)<1, whereas at Aw of 1 it is considered moist heat sterilization. Dry heat sterilization is typically conducted at 160- 170 oC. Its major purpose is the simultaneous achievement of bioburden sterilization and endotoxin inactivation. In general sterilization procedures and endotoxins inactivation is difficult to achieve [50]. Although it is possible to inactivate lipopolysaccharide, which constitute endotoxins [50], such treatment also damages the material itself, making it unacceptable for maintaining material and functional compatibility. This concern is emphasized in ISO TC 198 documents, which reference the ISO 9000 series (e.g., 25,32). Endotoxin can be inactivated by dry heating at 250 oC for 1h, resulting in a 4-log reduction [50]. Materials also to withstand this treatment are limited to stainless steel, glass and ceramics. Sterilization validation requires the inactivation of gram-negative bacteria. Simultaneous inactivation of endotoxins and gram-negative bacteria is required under GMP. Therefore, both sterilization validation requirements and GMP requirements must be met. However, in practical sterilization validation, there is no requirement to inactivate endotoxins. Even if endotoxin inactivation of endotoxins is not achieved, sterilization validation is still considered successful if the required SAL such as 10-6 is attained. Nevertheless, this requirement does not fully meet GMP expectations and therefore ISO 20857 may need to be revised [49]. The BI used for dry heat sterilization is B. atrophaeus ATCC 9372, a gram-positive bacterium [51].
The mechanism of dry heat sterilization is heating conduction. The efficiency of heat conduction under dry conditions is limited because air is a poor conductor of heat. Microorganisms exposed to dry heating are damaged and sterilized by air oxidation. Dry heat sterilization requires relatively high temperature typically above 170 oC and the sterilization period is prolonged. In contrast, the mechanism of moist heat sterilization is protein denaturation within spores due to the penetration of pressurized steam [35]. The sterilization period for moist heat sterilization is shorter than that of dry heat. The BI used for moist heat sterilization is G. stearothermophilus ATCC 7953 [27], whereas that used for dry heat sterilization is B. atrophaeus ATCC 9372 [51], which is identical to that used for EOG sterilization [28]. Several factors affecting dry heat sterilization still need to be clarified. For example, presence of antioxidant such as BHT, ascorbate, tocopherol and hindered amine may interfere with its efficacy, since the mechanism is based on air oxidation. Dry heat sterilization is typically conducted at 170 oC. D-value ranges from 8 seconds to 1.5 minutes, representing an 11- fold difference. An FH based on a Z-value of 20 oC is obtainable, which is the analogous to the concept of Fo in moist heat sterilization [38]. The mechanism of endotoxin inactivation by dry heat sterilization is complex and this has not yet been fully clarified. Current evidence suggests that inactivation occurs as a secondary reaction and requires a temperature above 250 oC [50].
The D-value for endotoxin inactivation at 170 oC is 20 minutes [50]. From this D-value the Z-value is calculated to be approximately 40 oC [38]. In dry heat sterilization validation, F170 oC is used as FH with a Z value of 20 oC [38]. Typically, Z values ranged from 13 oC to 28 oC. It is rare to utilize dry heat sterilization as the final sterilization procedure: in most cases, it is applied to sterilize intermediate materials. Typically, the major purpose is endotoxin inactivation along with the simultaneous sterilization of bioburden on glass vessels, ampoules and vials prior to aseptic filling or sealing. According to ISO 11138-4, a labeled D-value of >2.0 minutes at 160 oC is required [51]. Generally, the D-value at 170 oC is about 1.5 minutes, however the D-value of BI placed in the cold spots of practical sterilizers often exceeds 2.5 minutes, which is consistent with the ISO 11138-4 requirement. Notably, the temperature distribution within the chamber during heating should be uniform, indicating no cold spots should exist. The identification of cold spots and confirmation of their reproducibility must be validated. For the validation studies of endotoxin inactivation inoculated, the inoculated BI method is recommended requiring more than a 3-log reduction [49-51].
Conclusion
The authors conclude that validation studies must be conducted prior to the routine control in accordance with ISO requirements. These requirements are emphasized not only in ISO standards, but also in GMP, HACCP and related guidelines. Sterility-related documents developed under ISO TC 198 have resulted in several reliable ISO standards, and it is desirable to follow them. Doing so allow for the successful simultaneous achievements of both validation and routine control.
Conflict of Interests
The authors conclude that validation studies must be conducted prior to the routine control in accordance with ISO requirements. These requirements are emphasized not only in ISO standards, but also in GMP, HACCP and related guidelines. Sterility-related documents developed under ISO TC 198 have resulted in several reliable ISO standards, and it is desirable to follow them. Doing so allow for the successful simultaneous achievements of both validation and routine control.
References
- (1997) In: Furuhasi S, Shintani H (Eds.), Sterilization procedures, sterilization validation and sterility assurance of health care products based on ISO documents. Japan Standards Association, Tokyo, Japan.
- (1998) In: Shintani H (Ed.), Sterilization validation of pharmaceuticals and medical devices. Jihou Inc, Tokyo, Japan.
- (2010) In: Shintani H (Ed.), Guide to sterilization validation practice of pharmaceuticals and medical devices. Information Association, Tokyo, Japan.
- (1998) In: Shintani H (Ed.), Medical device GMP and sterilization validation of medical devices and pharmaceuticals. Technical Information Association, Tokyo, Japan.
- Shintani H (2011) Validation of sterilization procedures and usage of biological indicators in the manufacture of healthcare products. Biocontrol Sci 16(3): 85-94.
- Shintani H (2012) Validation study and routine control monitoring of moist heat sterilization procedures. Biocontrol Sci 17(2): 57-67.
- Shintani H (2014) Important points to attain reproducible sterility assurance. Biochem Physiol 3: 135-141.
- Shintani H (2009) Moist heat sterilization validation. J Antibacteria Antifungal Agents 37(8): 581-593.
- (2016) In: Shintani H, Sakudo A (Eds.), Gas plasma sterilization in microbiology: Theory, applications, pitfalls and new perspectives. Caister Academic Press, Poole, UK.
- Shintani H, Sakudo A, Burke P, Donnell GM (2010) Gas plasma sterilization of microorganisms and mechanism of action. Exp Ther Med 1(5) 731-738.
- Shintani H (2017) Ethylene oxide gas sterilization of medical devices. Biocontrol Sci 22(1): 1-16.
- Shintani H, Akers JE (2000) On the cause of performance variation of biological indicator used for sterility assurance. PDA J Pharm Sci Technol 54(4): 332-342.
- Shintani H (2015) Validation study of nitrogen gas plasma exposure based on ISO documents (mainly ISO TC 198 and 194 documents). Pharmaceut Regulatory Affairs 4: e150.
- Shintani H (1999) On attainment of reproducible sterility assurance demanded by ISO TC 198. J Med Ins 69: 183-189.
- (2010) In: Shintani H (Ed.), Practical aspect of sterilization and disinfection at production site of pharmaceuticals and medical devices-validation practice and routine monitoring. Technical Information Association, Tokyo, Japan.
- Shintani H (2015) Validation study on how to avoid microbial contamination during pharmaceutical production. Biocontrol Sci 20(1): 1-10.
- (2012) In: Shintani H (Ed.), Technique of sterilization, disinfection, decontamination, inactivation to remove hazardous matters. Sci Technol A13.
- Shintani H (2000) Sterility assurance of healthcare products-mainly on moist heat sterilization. PDA J, Japan, 2: 53-67.
- Shintani H, Kazuma K (2005) Several aspects of biological indicators for sterility assurance. Biocontrol Sci 10(4): 121-130.
- Shintani H, Taniai E, Miki A, Kurosu S, Hayashi F (2004) Comparison of the collecting efficiency of microbiological air samplers. J Hosp Infect 56(1): 42-48.
- Shintani H (2013) Importance considering increased recovery of injured microorganisms to attain reproducible sterilization validation. Pharm Anal Acta 4(1): 1-5.
- ISO 11138-1 (2018) Sterilization of health care products-Biological indicators-Part 1: General requirement.
- ISO 11737-1 (2018) Sterilization of health care products-Microbiological methods-Part 1: Determination of a population of microorganisms on products.
- ISO 14161 (2009) Sterilization of health care products-Biological indicators-Guidance for the selection, use and interpretation of results.
- ISO 9001 (2018) Quality management systems-Requirements.
- ISO 11138-7 (2019) Sterilization of health care products-Biological indicators-Part 7-Guidance for the selection, use and interpretation of results.
- ISO 11138-3 (2018) Sterilization of health care products-Biological indicators-Part 3-Biological indicators for moist heat sterilization processes.
- ISO 11138-2 (2018) Sterilization of health care products-Biological indicators-Part 2-Biological indicators for ethylene oxide sterilization.
- Shintani H, Sasaki K, Kajiwara Y, Itoh J, Takahashi M, et al. (2000) Validation of D value by different SCD culture medium manufacturer and/or different culture medium constituent. PDA J Pharm Sci Technol 54(1): 6-12.
- ISO 11137 (2018) Sterilization of health care products-Radiation-Requirements for development, validation and routine control of a sterilization process for medical devices.
- Sasaki K, Shintani H, Itoh J, Kamogawa T, Kajiwara Y (2000) Effect of calcium in assay medium on D value of Bacillus stearothermophilus ATCC 7953 spores. Appl Environ Microbiol 66(12): 5509-5513.
- ISO 13485 (2018) Medical devices-Quality management systems-Requirements for regulatory purposes.
- ISO 11135 (2018) Medical devices-Validation and routine control of ethylene oxide sterilization.
- ISO 10993-7 (2018) Biological evaluation of medical devices-Part 7: Ethylene oxide sterilization residuals.
- ISO 17665 (2018) Sterilization of health care products-Moist heat-Requirement for the development, validation and routine control of a sterilization process for medical devices.
- ICH-Q6A (1999) (Specifications: Test procedure and acceptance criteria for new drug products: Chemical substances) Agreement of guideline ICH (Step 4).
- (2002) On new drug regulation and test method procedure. Issued by the Medical Examination Board 568th.
- Shintani H (2003) On calculation of D value, Z value, L value, F value, Fo value and FH J Antibact Antifung Agent 31: 557-560.
- ISO 11140 (2014) Chemical indicator.
- Shintani H (1995) The relative safety of gamma-ray, autoclave and ethylene oxide gas sterilization of thermosetting polyurethane. Biomed Instrum Technol 29(6): 513-519.
- (2000) In: Booth FA (Ed.), Sterilization validation and routine operation handbook-Ethylene oxide. Technomic publishing Co Inc, Lancaster, USA.
- JIS Z-8806 (2001) Humidity-determination method.
- Shintani H, Yamase Y, Yamaguchi T (2005) Ethylene oxide gas sterilization procedure and sterilization validation method. J Antibact Antifung Agent 33: 137-149.
- Shintani H, Tahata T, Hatakeyama K, Takahashi M (1995) Comparison of D10 value accuracy by the Limited Sperman-Karber Procedure (LSKP), the Stumbo-Murphy-Cochran Procedure (SMCP) and the survival curve method (EN). Biomed Instrum Technol 29(2): 113-124.
- (2001) In: Fairand BP (Ed.), Radiation sterilization for health care product. CRC Press, NY, USA.
- (2001) In: Booth AF (Ed.), Sterilization validation and routine operation handbook-Radiation. CRC Press, Boca Raton, USA.
- Guideline for the safety test by Ministry of Health & Labor Welfare (2001).
- Shintani H, Suzuki E, Sakurai M (2003) Determination of compounds inhibiting bacterial growth in sterilized medical devices. Chromatographia 58: 193-199.
- ISO 20857 (2022) Sterilization of health care products-Dry heat-Requirement for the development, validation and routine control of a sterilization process for medical devices.
- (2007) In: Williams KL (Ed.), Endotoxins. Informa healthcare USA Inc, NY, USA.
- ISO 11138-4 (2018) Sterilization of health care products-Biological indicators, Part 4-Biological indicators for dry heat sterilization.
© 2025 Hideharu Shintani, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






