- Submissions

Full Text
Research & Investigations in Sports Medicine
Efficacy and Safety of Blend of Beetroot and Pomegranate Extract on Strength, Endurance, Nitric Oxide Bioavailability and Body Composition: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial in Healthy Active Males
Harshith Chandra Shekhar1, Lincy Joshua2 and Jestin V Thomas2*
1BGS Global Institute of Medical Sciences, BGS Health and Education City, Karnataka, India
2Leads Clinical Research and Bio Services Pvt. Ltd., Karnataka, India
*Corresponding author:Jestin V Thomas, Leads Clinical Research and Bio Services Pvt. Ltd., Karnataka, India
Submission: January 10, 2025;Published: January 26, 2026

ISSN: 2577-1914 Volume11 Issue 4
Abstract
Both nitrate and polyphenol pathways are known to support vascular function, muscle oxygenation, and endurance. A novel blend of Beetroot and Pomegranate Peel Extract (BPE) standardized for nitrates and ellagic acid content. This randomized, double-blind, placebo-controlled, two-period cross-over study evaluated the acute and 28-day effects of BPE in 18 healthy, physically active men on Nitric Oxide (NO) bioavailability, exercise performance, endurance and recovery. Participants received BPE (400mg/day) or placebo for 28 days with a 14-day washout. Compared with placebo, BPE produced significant improvements in bench press one-repetition maximum (Day 28: 9.44±8.73kg vs 2.22±8.09kg; p=0.0146), leg press one-repetition maximum (13.06±9.10kg vs 4.72±9.15kg; p=0.0097), dominant hand grip strength (3.28±2.88kg vs 1.01±1.98kg; p=0.0094), and six-minute walk distance (41.11±17.45m vs 12.22±17.68m; p<0.0001). Peak NO (p=0.0056) and average NO (p=0.0170) increased significantly with BPE, while perceived exertion decreased (−0.72±0.83 vs 0.17±1.04; p=0.0079). Fat mass and total body fat percentage were significantly reduced (p=0.0002 and p=0.0016, respectively) without loss of lean mass. SmO₂ improved within groups but showed no significant between-group differences. BPE was well tolerated, with no serious or product-related adverse events. These findings demonstrate acute and sustained ergogenic benefits of combined nitrate and ellagic acid supplementation in healthy active men..
Keywords: BPE; Beetroot extract; Pomegranate extract; Nitric oxide; Endurance performance; Strength; Nitrate supplementation; Ergogenic aid; Body composition; Skeletal muscle oxygenation
Abbreviations: 1-RM: One-Repetition Maximum; 6-MWT: Six-Minute Walk Test; AE: Adverse Event; BMI: Body Mass Index; DEXA: Dual-Energy X-Ray Absorptiometry; NO: Nitric Oxide; NOS: Nitric Oxide Synthase; RPE: Rate of Perceived Exertion; SAE: Serious Adverse Event; SE: Standard Error; SmO₂: Skeletal Muscle Oxygenation
Introduction
Ergogenic aids, including nutritional, mechanical, pharmacological, and psychological strategies, are widely utilized to enhance exercise performance, facilitate recovery, and optimize adaptation to training while maintaining overall health [1,2]. The global sport and exercise nutrition market has shown substantial growth, valued at approximately USD 40-45 billion in 2021 and projected to expand at an annual rate of around 8% through 2030, reflecting increasing interest in evidence-based nutritional interventions that support physical performance, recovery, and body composition [3,4]. Endurance capacity represents a critical determinant of physical fitness, reflecting the efficiency of the circulatory and respiratory systems in sustaining prolonged exercise [5]. Athletes participating in endurance-based disciplines such as running, triathlon, cycling, swimming, and rowing depend heavily on their aerobic capacity and metabolic efficiency to sustain optimal performance [6,7]. A variety of nutritional interventions, both macronutrient- and micronutrient-based, have been investigated for their ergogenic potential [1]. Among these, dietary nitrates have gained significant attention due to their promising effects on cardiovascular efficiency and exercise performance. Major dietary sources of nitrates include beetroot juice, pomegranate extract, and green leafy vegetables such as lettuce, spinach, and collard greens [8,9].
Upon ingestion, dietary nitrate is sequentially reduced to nitrite and then to Nitric Oxide (NO). Although NO is primarily synthesized endogenously from L-arginine through Nitric Oxide Synthase (NOS), the nitrate-nitrite-NO pathway provides an alternative and complementary mechanism for NO production, especially under hypoxic or acidic conditions [10,11]. NO plays a pivotal role in skeletal muscle physiology by modulating vascular tone, mitochondrial respiration, calcium handling, and glucose uptake, thereby facilitating improved oxygen delivery and utilization during exercise [10-13]. Several systematic reviews and meta-analyses have reported that dietary nitrate supplementation enhances exercise efficiency, delays fatigue, and improves endurance performance in both trained and untrained individuals [12,13]. Beetroot juice, a natural source of inorganic nitrate (NO3-), has been widely investigated for its ergogenic potential. NO generated from dietary nitrate promotes vasodilation, enhances blood flow, and improves mitochondrial efficiency, collectively contributing to greater cardiorespiratory endurance, prolonged time to exhaustion and improved exercise efficiency [14-18]. Research also suggests that beetroot supplementation can modulate oxygen utilization during sub-maximal exercise and enhance tolerance to highintensity activity, particularly in recreational athletes [8,19-21].
Doses ranging from 140 to 280mL of beetroot juice have been shown to improve performance outcomes, with benefits reaching a threshold beyond approximately 8.4mmol of nitrate [21]. Accordingly, major sports nutrition authorities, including the Academy of Nutrition and Dietetics and the Australian Institute of Sport, recognize beetroot juice as a potentially effective ergogenic aid for enhancing endurance performance [22]. Pomegranate extract is another natural supplement of interest owing to its rich polyphenolic and antioxidant composition, particularly ellagitannins and ellagic acid, which possess anti-inflammatory, cardioprotective, and vasodilatory properties [23,24]. These bioactive compounds may support enhanced endothelial function and greater exercise tolerance. In a randomized, double-blind, placebo-controlled cross-over study involving active adults, acute supplementation with pomegranate extract significantly increased vessel diameter and post-exercise blood flow, while also extending time to exhaustion at 90-100% of peak velocity [25]. Similarly, a trial in trained cyclists reported that 15 days of pomegranate extract supplementation enhanced time to exhaustion and delayed the onset of ventilatory threshold, without adversely affecting recovery markers [26].
These findings indicate that polyphenols derived from pomegranate may offer both vascular and ergogenic benefits, thereby potentially complementing nitrate-based interventions. BPE is a novel formulation of homogenized extracts of beetroot standardized for nitrate content and pomegranate standardized for ellagic acid. The formulation is designed to enhance exercise performance and endurance by promoting NO-mediated vasodilation, improving muscle oxygenation, supporting strength and recovery, and augmenting energy metabolism. An unpublished randomized, double-blind, placebo-controlled, human clinical study in 21 healthy adults aged 20-35 years supplemented with BPE at a dose of 500mg and 1000mg doses demonstrated significant increase in flow-mediated dilation, as measured through brachial artery diameter and blood flow velocity at 1- and 2-hours post-dose on Days 1 and 7. Present study explores efficacy and safety of BPE as measured by exercise performance, endurance, NO bioavailability, and body composition in healthy active male participants.
Materials and Methods
Study design and overview
This prospective, randomized (1:1), double-blind, placebocontrolled, two-arm, two-period cross-over clinical study evaluated the efficacy and safety of BPE versus placebo in healthy adult males. The clinical phase for each participant lasted approximately 72 days and included a 2-day screening period, two 28-day treatment periods, and a 14±2-day washout period between treatment phases. The study was initiated after obtaining written approval from an institutional ethics committee of BGS Global Institute of Medical Sciences, Bangalore, India. The study was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki, the New Drugs and Clinical Trials Rules, 2019, and applicable local regulations. The study was registered with the Clinical Trials Registry of India (CTRI/2025/05/087113).
Eligibility criteria
Healthy, physically active male participants meeting all inclusion criteria and none of the exclusion criteria were considered eligible for enrolment in the study. Subjects who met all of the following criteria were included in the study. Healthy and physically active male volunteers aged between 18 and 35 years (both limits inclusive), with a Body Mass Index (BMI) between 18.5 and 29.9kg/m², were eligible to participate. Subjects were required to be free of any physical limitations or chronic illness that could affect performance, not on any medications, and not consuming supplements containing anabolic agents or protein powders within the past four weeks. Eligible participants were those who agreed to abstain from alcohol for at least 24 hours and caffeine or caffeinecontaining products for 12 hours before each visit, and to refrain from vigorous physical activity or strenuous exercise for 24 hours prior to test days. Participants were also required to provide written informed consent and to be willing and able to understand and comply with all study requirements, including consumption of the investigational product as instructed, attending scheduled visits, adhering to study restrictions, and completing the study. Subjects who met any of the following criteria were excluded from the study.
Individuals with known hypersensitivity or a history of allergy to the study product, or those presenting with uncontrolled hypertension (systolic blood pressure >160mmHg or diastolic blood pressure >100mmHg) at screening, were not eligible. Subjects on any medications, or with malignant disease, end-stage organ disorders, or laboratory abnormalities deemed clinically significant by the investigator, were excluded. Participants with metabolic disorders such as uncontrolled diabetes or thyroid dysfunction, or those suffering from severe chronic diseases including cancer, renal failure, HIV, immunodeficiency, hepatic or biliary disorders, arthritis, or uncontrolled cardiac conditions were also excluded. Any medical condition that could adversely impact participant safety or confound study results was considered exclusionary. In addition, individuals with a history of drug or alcohol abuse, or those who had received any investigational drug or device within the three months prior to study entry, were excluded from participation.
Study procedures
The subjects were enrolled in the study only after providing written informed consent, following a detailed verbal and written explanation of the study objectives, procedures, potential risks and participant responsibilities during the screening period (Day −2 to Day 0), with adequate time provided to address any questions or concerns. Demographic data, medical history, concomitant medications and physical examinations were recorded. Safety laboratory tests performed at screening included complete blood count, liver function tests [alanine transaminase and aspartate transaminase] and renal function tests [serum creatinine and blood urea nitrogen]. Maximal strength testing (one-Repetition Maximum, 1-RM) for leg press and bench press was completed at screening to determine individualized exercise loads.
Randomization and blinding
After confirmation of eligibility, subjects were randomized in a 1:1 ratio to one of two treatment sequences (Sequence 1: placebo → test product; Sequence 2: test product → placebo) using a computer-generated randomization schedule. Each randomized subject received a unique randomization number. Blinding was maintained by dispensing identically appearing coded capsules; dispensing was performed by an independent dispenser who had no role in outcome assessments. Investigators, site staff involved in efficacy/safety assessments, and participants remained blinded to treatment allocation throughout the study.
Investigational product and dosing
The investigational product, BPE, is a homogenized hydroalcoholic extracts of beetroot and pomegranate peel, respectively standardized for 2.5% nitrates and 10% ellagic acid (commercially known as BeepActiveTM), as measured by validated High- Performance Liquid Chromatography (HPLC). BPE is provided as 500mg (400mg BPE and 100mg of microcrystalline cellulose) oral capsule. The placebo contained 500mg microcrystalline cellulose. Both the investigational product and placebo were manufactured by Samriddh Nutractive Private Limited, India. Participants were instructed to consume one capsule daily each morning before breakfast for 28 days during each supplementation period.
Study visits and procedures
Participants attended five clinic visits: screening/visit 1 (Day- 2 to 0), baseline 1/visit 2 (Day 1), follow-up/visit 3 (Day 28±2), cross-over/baseline 2/visit 4 (Day 42±2), and end of study/visit 5 (Day 70±2). At each scheduled baseline visit (pre-dose) and at the scheduled post-dose time point (1 hour after supervised ingestion at the clinic), assessments were performed as applicable. These included body composition measurement by Dual-Energy X-Ray Absorptiometry (DEXA) for body fat percentage, body fat mass and lean body mass; evaluation of muscle strength using 1-RM leg press (quadriceps strength) and bench press (pectoral strength); hand grip strength assessment with a hand-held dynamometer (Camry EH101, China); and endurance assessment using the 6-minute treadmill walk test to determine total distance walked. NO bioavailability and skeletal muscle oxygenation (SmO2) were measured with the NNOXX device during the treadmill test, and the Rate of Perceived Exertion (RPE) was recorded using the Modified Borg Scale. On Days 1, 28, 42, and 70, post-dose testing was performed at 60 minutes after supervised ingestion to capture acute effects except for DEXA which was done only at Day 28.
Safety monitoring
Safety assessments included physical examinations, vital signs (seated blood pressure, heart rate, and temperature after 5 minutes of rest), laboratory evaluations, and monitoring for Adverse Events (AEs) and Serious Adverse Events (SAEs).
Outcomes and endpoints
The primary efficacy endpoint was mean change from baseline in 1-RM muscle strength (leg press and bench press) comparing BPE to placebo on Day 1 (acute) and Day 28 (sub-acute). Secondary endpoints included change from baseline in hand grip strength, endurance (6-minute treadmill walk distance), NO and SmO2 responses during exercise, RPE (Modified Borg Scale), and body composition (DEXA). Safety endpoints encompassed clinically significant changes in laboratory parameters, incidence of AEs/ SAEs, and changes in vital signs and physical examination findings.
Sample size determination
Eighteen subjects were enrolled to achieve at least 15 evaluable participants, which was calculated to provide 80% power to detect a clinically meaningful difference at a two-sided alpha level of 0.05 assuming a 5% dropout rate.
Statistical analysis
All statistical analyses were conducted using R statistical software. Demographic and baseline characteristics, including age, height (cm), weight (kg), and BMI (kg/m²), were summarized and tabulated by randomized treatment group and for the overall study population. All analyses were performed on the randomized population. Continuous variables were summarized using mean, Standard Error (SE), median, minimum, and maximum. Categorical variables were summarized using frequency counts and percentages. Normality of dependent variables was assessed with the Shapiro–Wilk test; parametric tests (independent t-tests for between-group comparisons and paired t-tests for within-group changes) were used for normally distributed data. Non-parametric alternatives were employed when normality assumptions were not met. Analyses were two-sided with alpha=0.05. Safety analyses were performed on the safety population (all randomized subjects who received any investigational product); efficacy analyses were performed on the randomized study completed population.
Efficacy and safety endpoints evaluation
The primary efficacy endpoint was the mean change from baseline in muscle strength parameters for BPE compared to placebo. This included the change in 1-RM strength, specifically quadriceps strength assessed by the leg press exercise and pectoral strength assessed by the bench press exercise, evaluated on Day 1 (1-hour post-dose) and Day 28. The secondary efficacy endpoints included the mean change from baseline in multiple performance and physiological parameters for BPE compared to placebo. These comprised changes in hand grip strength assessed using a handheld dynamometer on Day 1 (1 hour post-dose) and Day 28; endurance evaluated by the Six-Minute Walk Test (6-MWT) on Day 1 (1 hour post-dose) and Day 28; NO and SmO2 measured during the 6-minute treadmill walk test on Day 1 (1 hour post-dose) and Day 28; RPE assessed by the Modified Borg Scale on Day 1 (1 hour postdose) and Day 28; and changes in body composition parameters, including body fat percentage, body fat mass and lean body mass, assessed by DEXA on Day 28. The safety endpoints for the study included clinically significant changes from baseline to the end of treatment in laboratory parameters, the incidence of AEs and SAEs, and variations in vital sign parameters.
Results
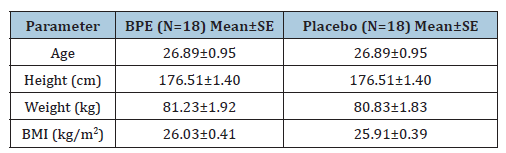
A total of 23 subjects were screened for participation in the study, of whom 18 met the eligibility criteria and were randomized to receive BPE and placebo in a cross-over design (Figure 1). All 18 randomized subjects completed both treatment periods and were included in the efficacy and safety analyses. There were five screen failures, and no subjects discontinued or withdrew after randomization. The mean (±SE) age of the subjects enrolled in the study was 26.89±0.95 years in both groups (Table 1). All participants were Indian-origin males. The mean (±SE) height in the BPE and placebo groups was 176.51±1.40cm, with mean (±SE) body weights of 81.23±1.92kg and 80.83±1.83kg, respectively. The mean (±SE) BMI was 26.03±0.41kg/m2 and 25.91±0.39kg/m2, respectively. Overall, the demographic and baseline characteristics, including age, anthropometric measurements, and vital parameters, were comparable between the groups and within normal ranges throughout the study.
Figure 1:CONSORT diagram of participant flow.
Table 1:Demographic and anthropometric parameters of subjects at Day 1.
BMI: Body mass index; N: Number of subjects; SE: Standard error.
Primary efficacy endpoints
Muscle strength (1-RM) - Bench Press (Pectoral Strength): As illustrated in Figure 2a, within-group analysis of the 1-RM bench press test indicated that BPE produced statistically significant increases (p<0.05) in both the acute and sub-acute phase compared to baseline. The mean (±SE) change from baseline to Day 1 postdose (1 hour after ingestion) was 5.83±2.07kg (p=0.0119), while the change from baseline to Day 28 was 9.44±2.06kg (p=0.0003). In contrast, the placebo group demonstrated non-significant changes of 1.67±1.81kg at Day 1 post-dose (p=0.3695) and 2.22±1.91kg at Day 28 (p=0.2596). Between-group analysis of mean change from baseline showed that the increase at Day 28 (9.44±2.06kg vs 2.22±1.91kg; p=0.0146) was significantly greater (p<0.05) with BPE compared to placebo, whereas increase at Day 1 post-dose (5.83±2.07kg vs 1.67±1.81kg; p=0.1390) did not reach statistical significance between the two groups. Percentage increases for BPE were 7.49% at Day 1 and 12.64% at Day 28 compared with 2.45% and 4.05% for placebo, confirming both acute and sub-acute improvements in pectoral strength.
Figure 2:Average change from baseline in bench press 1-RM (kg) (a) and leg press 1-RM (kg) (b) by treatment and visit. Bars indicate mean±SE.
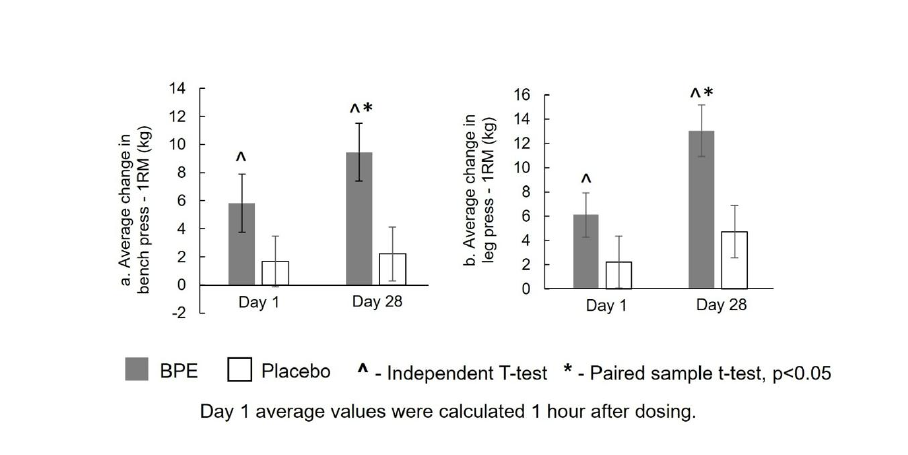
Muscle strength (1-RM) - Leg Press (Quadriceps Strength): As shown in Figure 2b, within-group analysis of the 1-RM leg press test demonstrated statistically significant increases (p<0.05) in both acute and sub-acute performance in the BPE group. The mean (±SE) change from baseline to Day 1 post-dose (1 hour after ingestion) was 6.11±1.83kg (p = 0.0039), and from baseline to Day 28 was 13.06±2.14kg (p<0.0001). The placebo group showed a non-significant change of 2.22±2.15kg at Day 1 post-dose (p=0.3152) and a smaller but significant increase of 4.72±2.16kg at Day 28 (p=0.0428). Between-group analysis of mean change from baseline demonstrated a statistically significant increase at Day 28 (13.06±2.14kg vs 4.72±2.16kg; p=0.0097) favouring BPE, while the increase at Day 1 post-dose (6.11±1.83kg vs 2.22±2.15kg; p=0.1776) was not statistically significant between the groups. Percentage increases for BPE were 4.20% at Day 1 and 9.40% at Day 28 compared with 1.43% and 3.46% for placebo, confirming both acute and sub-acute improvements in quadriceps strength.
Secondary efficacy endpoints
Hand grip strength (hand-held dynamometer) - Dominant hand grip strength: Dominant and non-dominant hand grip strength were assessed. The results are illustrated in Figure 3. Within-group analysis of dominant hand grip strength for BPE showed no significant change from baseline to Day 1 postdose (mean±SE: 1.01±0.74kg; p=0.1887), but demonstrated a significant (p<0.05) increase from baseline to Day 28 (3.28±0.68kg; p=0.0001). These changes corresponded to percentage increases of 2.86% and 8.44%, respectively, for acute and sub-acute effects. In contrast, the placebo group showed no improvement at Day 1 postdose (−0.05±0.45kg; p=0.9048) and a mild but significant (p<0.05) change at Day 28 (1.01±0.46kg; p=0.0446), corresponding to percentage increases of 0.08% and 2.79%, respectively. Betweengroup analysis of mean change from baseline indicated that the increase at Day 28 (3.28±0.68kg vs 1.01±0.46kg; p=0.0094) was significantly greater (p<0.05) with BPE compared to placebo, whereas the increase at Day 1 post-dose (1.01±0.74kg vs −0.05±0.45kg; p=0.2273) was not statistically significant between the two groups. Percentage increases for BPE (2.86% at Day 1 and 8.44% at Day 28) compared with placebo (0.08% and 2.79%) confirm a clear sub-acute enhancement in dominant hand strength.
Figure 3:Average change from baseline in Hand grip strength (dominant and non-dominant hands) by treatment and visit (kg). Mean±SE.
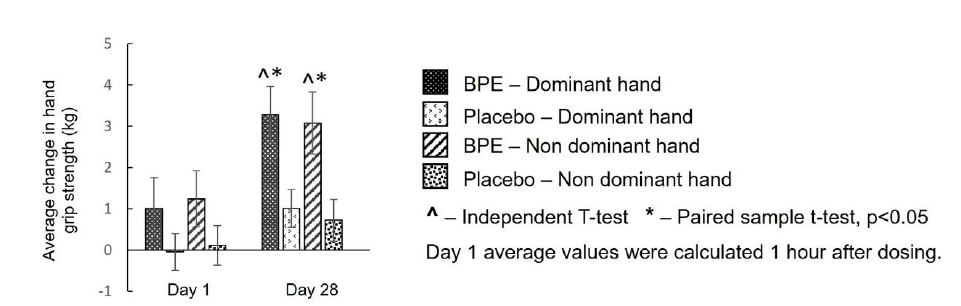
Hand grip strength (hand-held dynamometer)-nondominant hand grip strength: Within-group analysis of nondominant hand grip strength for BPE demonstrated a non-significant increase from baseline to Day 1 post-dose (mean±SE: 1.24±0.68kg; p=0.0875) and a significant (p<0.05) improvement from baseline to Day 28 (3.08±0.75kg; p=0.0008). These corresponded to percentage increases of 3.52% and 8.84%, respectively, for acute and sub-acute changes. In contrast, the placebo group showed non-significant changes at both time points, with mean (±SE) differences of 0.11±0.48kg at Day 1 (p=0.8143) and 0.72±0.51kg at Day 28 (p=0.1786), corresponding to percentage increases of 0.45% and 2.33%, respectively. Between-group analysis of mean change from baseline showed that the increase at Day 28 (3.08±0.75kg vs 0.72±0.51kg; p=0.0145) was significantly greater (p<0.05) with BPE compared to placebo, whereas the increase at Day 1 post-dose (1.24±0.68kg vs 0.11±0.48kg; p=0.1897) was not statistically significant between the groups. Percentage increases for BPE (3.52% at Day 1 and 8.84% at Day 28) compared with placebo (0.45% and 2.33%) support the conclusion of sub-acute improvement in non-dominant hand strength.
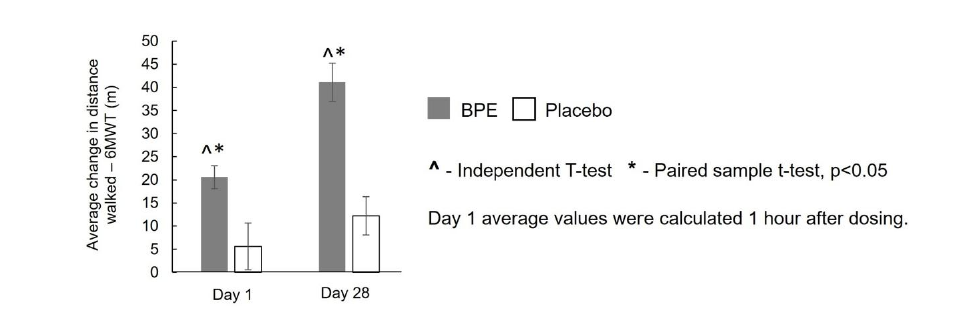
Endurance-6-Minute Treadmill Walk Test (6-MWT): As illustrated in Figure 4, within-group analysis of the 6-MWT results indicated that BPE produced statistically significant (p < 0.05) increases in both acute and sub-acute assessments compared to baseline. The mean (±SE) change from baseline to Day 1 postdose (1 hour after ingestion) was 20.56±2.49 meters (p<0.0001), while the change from baseline to Day 28 was 41.11±4.11 meters (p<0.0001), corresponding to percentage increases of 3.21% and 6.41%, respectively. In contrast, the placebo group demonstrated a non-significant change of 5.56±5.06 meters at Day 1 post-dose (p=0.2878) and a smaller but significant increase of 12.22±4.17 meters at Day 28 (p=0.0093), corresponding to percentage increases of 0.97% and 1.95%, respectively. Between-group analysis of mean change from baseline revealed that both the improvement at Day 1 post-dose (20.56±2.49m vs 5.56±5.06m; p=0.0135) and the improvement at Day 28 (41.11±4.11m vs 12.22±4.17m; p<0.0001) were significantly greater (p<0.05) with BPE compared to placebo, confirming substantial enhancements in endurance performance with BPE supplementation.
Figure 4:Average change from baseline in 6-Minute Walk Test (6-MWT) — distance (m) by treatment and visit. Mean±SE.
Nitric Oxide (NO) bioavailability - Peak NO: NO was reported as peak and average units measured during the 6-MWT. The results are presented in Figure 5a. Within-group analysis of peak NO levels demonstrated that BPE supplementation produced statistically significant (p<0.05) increases in both acute and sub-acute phases compared to baseline. The mean (±SE) change from baseline to Day 1 post-dose (1 hour after ingestion) was 7.56±3.33 units (p=0.0367), while the change from baseline to Day 28 was 25.89±4.65 units (p<0.0001), corresponding to percentage increases of 46.97% and 176.74%, respectively. In contrast, the placebo group showed no significant acute change (2.17±3.31 units; p=0.5209) and only a modest, non-significant increase at Day 28 (7.39±4.17 units; p=0.0944), corresponding to percentage increases of 24.52% and 47.82%, respectively. Between-group analysis indicated that the improvement at Day 28 (25.89±4.65 units vs 7.39±4.17 units; p=0.0056) was significantly greater (p<0.05) with BPE compared to placebo, whereas the change at Day 1 post-dose (7.56±3.33 units vs 2.17±3.31 units; p=0.2589) was not statistically significant between the groups.
Nitric Oxide (NO) bioavailability - Average NO: Withingroup analysis of average NO levels revealed a strong trend toward improvement at Day 1 post-dose (mean±SE: 4.77±2.41 units; p=0.0647) and a statistically significant (p<0.05) increase at Day 28 (18.29±4.12 units; p=0.0004), corresponding to percentage increases of 95.83% and 278.94%, respectively. The placebo group demonstrated non-significant changes in average NO both at Day 1 post-dose (1.52±2.50 units; p=0.5509) and Day 28 (5.28±3.12 units; p=0.1087), corresponding to percentage increases of 75.49% and 119.43%, respectively. Between-group analysis showed that the improvement at Day 28 (18.29±4.12 units vs 5.28±3.12 units; p=0.0170) was significantly greater (p < 0.05) with BPE, while the improvement at Day 1 post-dose (4.77±2.41 units vs 1.52±2.50 units; p=0.3570) was not statistically significant between the groups.
Figure 5:Average change from baseline in peak and average nitric oxide (NO) units (a) and SmO2 % (b) during 6-MWT by treatment and visit. Mean±SE.
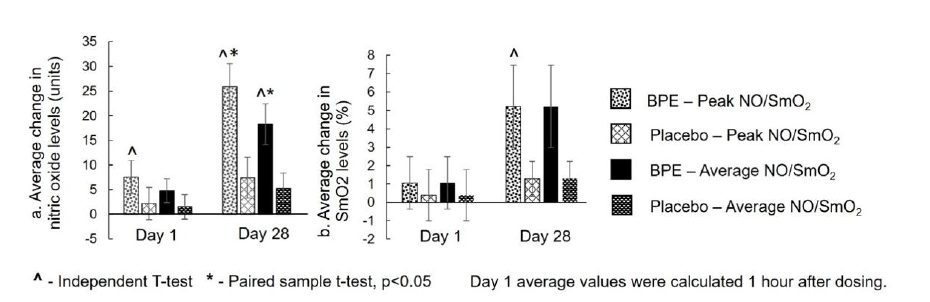
Skeletal Muscle Oxygenation (SmO2 %)-Peak SmO2: SmO2 % (peak and average; Figure 5b) measured during the 6-MWT increased numerically in the BPE arm. Within-group analysis of peak SmO2 levels indicated that BPE did not produce a significant change from baseline to Day 1 post-dose (mean±SE: 1.06±1.42%; p=0.4667), but demonstrated a statistically significant (p<0.05) improvement by Day 28 (5.22±2.24%; p=0.0323), corresponding to percentage increases of 2.24% and 9.66%, respectively. The placebo group showed no significant changes at either Day 1 postdose (0.39±1.41%; p=0.7853) or Day 28 (1.28±0.94%; p=0.1896), corresponding to percentage increases of 1.21% and 2.38%, respectively. Between-group comparison revealed that both the acute (5.22±2.24% vs 1.28±0.94%; p=0.1179) and sub-acute phase (1.06±1.42% vs 0.39±1.41%; p=0.7404) changes were not statistically significant between groups.
Skeletal Muscle Oxygenation (SmO2 %)-Average SmO2: Within-group analysis of average SmO2 levels showed no significant improvement from baseline to Day 1 post-dose (mean±SE: 1.06±1.42%; p=0.4966) or improvement from baseline to Day 28 (5.22±2.24%; p=0.2063) with BPE supplementation. The placebo group also showed non-significant changes at Day 1 post-dose (0.39±1.41%; p=0.9295) and Day 28 (1.28±0.94%; p=0.2419). Between-group comparisons revealed no statistically significant differences for either time points (Day 1: p=0.7219; Day 28: p=0.5328).
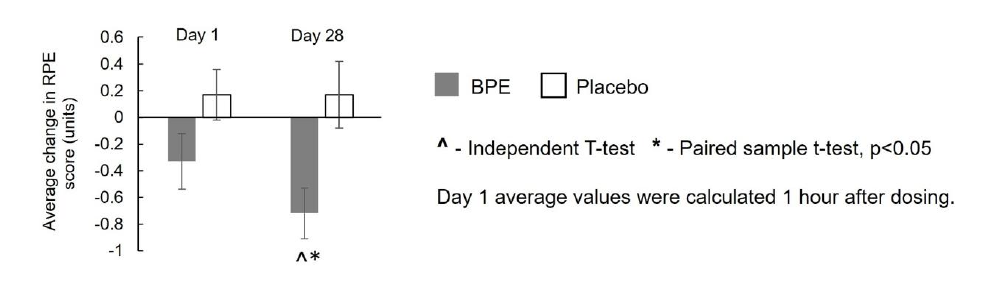
Rate of perceived exertion (modified borg scale): As illustrated in Figure 6, within-group analysis of RPE, assessed using the Modified Borg Scale, indicated that BPE supplementation did not produce a statistically significant reduction in perceived exertion from baseline to Day 1 post-dose (mean±SE:−0.33±0.21 units; p=0.1376), but resulted in a significant (p<0.05) reduction from baseline to Day 28 (−0.72±0.19 units; p=0.0017). These reductions corresponded to percentage decreases of 4.91% and 15.19%, respectively. In contrast, the placebo group showed small, non-significant increases in RPE at both time points (Day 1: 0.17± 0.19 units; p=0.3808; Day 28: 0.17±0.25 units; p=0.5070), corresponding to percentage increases of 6.34% and 6.47%, respectively. Between-group analysis of change from baseline revealed that the reduction in perceived exertion at Day 28 was significantly greater (p<0.05) with BPE compared with placebo (−0.72±0.19 vs 0.17±0.25; p=0.0079). Although the reduction at Day 1 post-dose (−0.33±0.21 vs 0.17±0.19; p=0.0864) favored BPE, it did not reach statistical significance. These findings suggest that BPE supplementation effectively reduced subjective exertion over 28 days, reflecting enhanced exercise tolerance and perceived effort regulation.
Figure 6:Average change from baseline in the rate of perceived exertion (Modified Borg Scale) by treatment and visit. Mean±SE.
Body composition (DEXA): Fat mass (g) As illustrated in Figure 7a, within-group analysis of fat mass showed that BPE supplementation produced a statistically significant reduction (p<0.05) from baseline to Day 28 (mean±SE: −921.44±220.21g; p=0.0006), corresponding to a percentage decrease of −2.66±0.65%. In contrast, the placebo group exhibited a non-significant increase (mean±SE: +141.83±118.36g; p=0.2472), corresponding to a percentage increase of +0.42 ± 0.36%. Between-group analysis of change from baseline demonstrated that the reduction in fat mass was significantly greater (p<0.05) in the BPE group compared with placebo (−921.44±220.21g vs +141.83±118.36g; p=0.0002). Lean body mass (g) As shown in Figure 7b, within-group analysis of lean body mass indicated that changes from baseline were nonsignificant in both groups (BPE: +249.72±181.89g, p=0.1876; Placebo: +62.17±39.36g, p=0.1327), corresponding to percentage increases of +0.58±0.40% and +0.14±0.09%, respectively. Betweengroup analysis revealed no statistically significant difference between BPE and placebo (249.72±181.89g vs 62.17±39.36g; p=0.3265).
Figure 7:Average body composition: fat mass (g), lean mass (g) and total fat % by treatment at baseline and Day 28. Mean±SE.
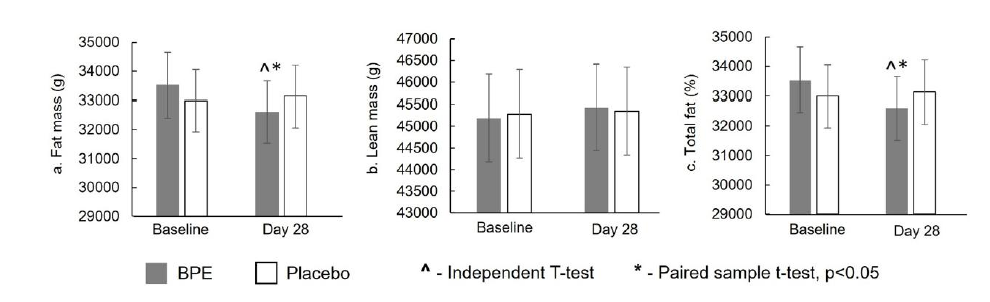
Total fat (%) As depicted in Figure 7c, within-group analysis of total fat percentage showed that BPE produced a statistically significant reduction (p<0.05) from baseline to Day 28 (mean±SE: −0.80±0.23%; p=0.0025), corresponding to a percentage decrease of −1.93±0.55%. In contrast, the placebo group exhibited a nonsignificant increase (+0.07±0.09%; p=0.4523), corresponding to a percentage increase of +0.18±0.23%. Between-group analysis of change from baseline demonstrated that the reduction in total fat percentage was significantly greater (p<0.05) with BPE compared with placebo (−0.80±0.23% vs +0.07±0.09%; p=0.0016). Overall, BPE produced clinically and statistically meaningful reductions in fat mass and percent body fat over 28 days without loss of lean mass.
Safety and tolerability
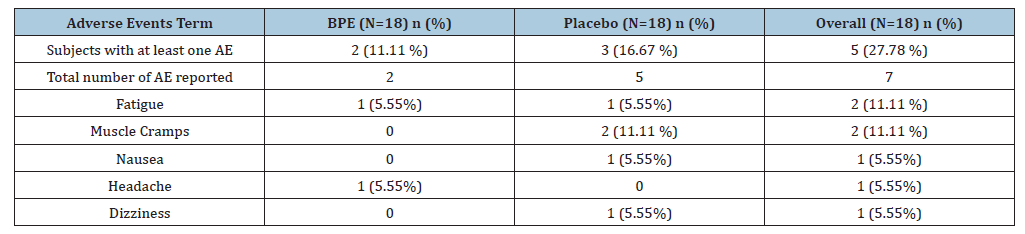
A total of seven AEs were reported during the study (Table 2). Two subjects (11.11%) in the BPE group and three subjects (16.67%) in the placebo group experienced at least one AE, representing an overall incidence of 27.78%. The total number of AEs reported was two in the BPE group and five in the placebo group. The most commonly reported AEs were fatigue and muscle cramps, each occurring in two subjects (11.11%) across both groups. Fatigue was observed in one subject (5.55%) from each group, while muscle cramps were reported only in the placebo group (11.11%). Other isolated events included nausea (5.55%) and dizziness (5.55%) in the placebo group, and headache (5.55%) in the BPE group. All reported AEs were mild, transient, and resolved without medical intervention. No SAE’s or clinically significant abnormalities were observed, and none were considered related to BPE supplementation.
Table 2:Details of Adverse Events (AE) experienced by subjects.
Discussion
This randomized, double-blind, placebo-controlled, two-period cross-over study demonstrated that BPE — a combination of standardized extract of beetroot and pomegranate peel produced meaningful improvements across multiple domains of exercise performance, physiological function, and body composition in healthy, physically active men. Compared to placebo, BPE yielded statistically significant long-term gains (Day 28) in upper- and lower-body maximal strength (bench press and leg press 1-RM), hand grip strength, endurance performance (6-MWT distance), and NO bioavailability. It also showed a trend toward improved SmO2, significant changes in RPE, and decreased fat mass and total body fat percentage without any loss of lean mass. BPE was well tolerated, with no serious or product-related AEs reported, confirming its safety for use in healthy adults. These findings are consistent with the biological rationale for combining inorganic nitrate from beetroot and polyphenol-rich pomegranate extract. Dietary nitrate is reduced through the sequential nitrate-to-nitrite-to-NO pathway, enhancing NO bioavailability particularly under hypoxic and acidic conditions. This increase in circulating and tissue NO contributes to improved vasodilation, muscle oxygenation, mitochondrial efficiency, calcium handling, and glucose uptake mechanisms that collectively enhance exercise tolerance and muscular efficiency.
Several meta-analyses have confirmed nitrate’s ergogenic potential, particularly in improving oxygen economy and timeto- exhaustion during endurance activity [8-16,19,20]. In the present study, BPE supplementation produced both acute (1- hour post-dose) and sub-acute (28-day) increases in NO levels, which were paralleled by significant improvements in endurance and strength parameters, reinforcing this mechanistic link. In parallel, pomegranate polyphenols, particularly ellagitannins and ellagic acid, have demonstrated antioxidant, anti-inflammatory, and endothelial-supporting properties that complement nitrate physiology. Polyphenols can stabilize NO and enhance endothelial NOS activity, improving blood flow and vascular responsiveness [23-26]. Previous trials have shown that pomegranate supplementation improves post-exercise vasodilation, vessel diameter, and time to exhaustion in athletes [25,26]. The present data showing increased NO and trends in SmO2 % together with improved perceived exertion are compatible with a synergy between nitrate-driven NO production and ellagic acid-mediated endothelial support, potentially enhancing both macrovascular and microvascular oxygen delivery and utilization during exercise [14-16,23-25,27,28]. Unlike some single-ingredient studies, the combined formulation here produced acute endurance benefit and durable improvements in strength and body composition over 28 days, suggesting additive or complementary effects.
The interaction between dietary nitrate and pomegranate polyphenols with relevance to nitric oxide mediated vascular and metabolic enhancement is possibly linked to the activation of the anti-aging gene Sirtuin 1 [29]. Sirtuin 1 is involved in vasodilation of the vasculature, metabolism and muscle function [30]. Nitric oxide, ellagic acid and punicalagin are Sirtuin 1 activators and exercise performance will be determined by Sirtuin 1 activators versus Sirtuin 1 inhibitors [31]. From a performance standpoint, BPE supplementation yielded statistically significant betweengroup differences versus placebo across most primary and secondary endpoints, including bench press and leg press strength, hand grip strength, endurance (6-MWT), and NO bioavailability. Improvements were accompanied by reduced perceived exertion and favourable changes in fat mass and total body fat percentage, without compromising lean mass. The observed reductions in fat mass and total body fat percentage following BPE supplementation may be attributed to the complementary physiological effects of nitrate-rich beetroot and ellagic acid-rich pomegranate extracts on metabolic regulation and vascular function.
Dietary nitrates enhance NO bioavailability, improving blood flow, mitochondrial efficiency, and skeletal muscle oxygen utilization, which collectively increase aerobic metabolism and energy expenditure [7-15,19]. Enhanced NO signalling also facilitates greater glucose and fatty acid uptake into skeletal muscle, promoting lipid oxidation during and after exercise [8,10- 15]. Pomegranate polyphenols, particularly ellagitannins and ellagic acid, exert antioxidant and anti-inflammatory effects that can improve endothelial function and reduce oxidative stress, further supporting mitochondrial function and metabolic flexibility [23–26]. Additionally, polyphenols have been shown to modulate lipid metabolism by downregulating lipogenic pathways and enhancing lipolytic activity [23-25]. Together, these mechanisms of BPE may contribute to improved substrate utilization, reduced adipose accumulation, and the favourable changes in fat composition observed in this study without compromising lean mass. Safety and tolerability outcomes were favourable. No SAEs were reported, and all recorded events were mild, transient, and unrelated to treatment. Laboratory and vital parameters remained within normal limits throughout the study, confirming the safety of BPE supplementation in healthy adult males. These findings align with prior reports demonstrating the safety of dietary nitrate and pomegranate extracts within similar dosing ranges [14,21,23].
Study strengths include the randomized, double-blind, crossover design, which reduces between-subject variability and allows within-subject comparisons of acute and sub-acute effects; objective, validated outcome measures (1-RM strength tests, DEXA, NNOXX NO/SmO2 assessments, and hand dynamometry); and preregistered endpoints with repeated acute and sub-acute assessments. The inclusion of both physiological (NO, SmO2) and functional (strength, 6-MWT, perceived exertion) outcomes enhances mechanistic interpretation. Nevertheless, the sample size was modest (n=18), and the study included only healthy young men in a single-center setting, which may limit generalizability to other populations such as women, older adults, or elite athletes. The 28-day duration reflects short-to-mid-term outcomes and does not address longterm efficacy, safety, or adaptation effects. Overall, these results support BPE as a promising, safe, and effective nutritional strategy for enhancing both strength and endurance capacity in active young adults, with simultaneous benefits on perceived exertion and fat reduction. For athletes and practitioners, BPE may offer dual-phase benefits-acute improvements following pre-exercise dosing and sustained gains with continued supplementation. Future studies should focus on larger, multi-center randomized controlled trials with longer follow-up duration to validate these findings, establish long-term efficacy and safety, and explore potential sex- and agespecific differences in response profiles.
Conclusion
In this randomized, double-blind, placebo-controlled cross-over study of healthy, physically active men, BPE produced rapid (acute) and sustained (28-day) improvements in endurance, muscular strength, NO bioavailability, perceived exertion, and body fat measures compared with placebo, while maintaining a favourable short-term safety profile. The overall pattern of physiological and performance benefits supports the synergistic interaction between dietary nitrate and pomegranate polyphenols, consistent with the underlying biology of NO-mediated vascular and metabolic enhancement. These findings also justify larger and longer trials to confirm efficacy, define optimal dosing, evaluate applicability to other populations, and further characterise mechanisms and longterm safety.
Acknowledgment
We thank all the study participants. Conceptualization, methodology, Harshith Chandra Shekhar and Jestin V. Thomas; software, validation, formal analysis, data curation, visualization Jestin V. Thomas and Harshith Chandra Shekhar; investigation, supervision, resources, Harshith Chandra Shekhar; writing-original draft preparation, project administration Harshith Chandra Shekhar and Lincy Joshua; writing-review and editing, Jestin V. Thomas. All authors have read and agreed to the published version of the manuscript. This research was funded by Samriddh Nutractive Pvt. Ltd. and The APC was funded by Samriddh Nutractive Pvt. Ltd.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kerksick CM, Wilborn CD, Roberts MD, Ryan AS, Kleiner SM, et al. (2018) ISSN exercise & sports nutrition review update: Research & recommendations. J Int Soc Sports Nutr 15(1): 38.
- Carey CC, Doyle L, Lucey A (2023) Nutritional priorities, practices and preferences of athletes and active individuals in the context of new product development in the sports nutrition sector. Front Sports Act Living 5: 1088979.
- (2025) Sports Nutrition Market Size Worth $82.3 Billion By 2030.
- Crimmins EM (2015) Lifespan and healthspan: Past, present, and promise. Gerontologist 55: 901-911.
- Pate R, Oria M, Pillsbury L, Committee on Fitness Measures and Health Outcomes in Youth, Food and Nutrition Board, et al. (2012) Health-Related Fitness Measures for Youth: Cardiorespiratory Endurance. Fitness Measures and Health Outcomes in Youth.
- Morici G, Auria CIG, Baiamonte P, Mazzuca E, Castrogiovanni A, et al. (2016) Endurance training: Is it bad for you? Breathe 12(2): 140-147.
- Lundberg JO, Govoni M (2004) Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radical Biol Med 37(3): 395-400.
- Clements WT, Lee SR, Bloomer RJ (2014) Nitrate ingestion: A review of the health and physical performance effects. Nutrients 6(11): 5224-5264.
- Hord NG, Tang Y, Bryan NS (2009) Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am J Clin Nutr 90(1): 1-10.
- Husmann F, Bruhn S, Mittlmeier T, Zschorlich V, Behrens M (2019) Dietary nitrate supplementation improves exercise tolerance by reducing muscle fatigue and perceptual responses. Front Physiol 10: 404.
- Calvo JL, Capo FA, Galeano HP, Jiménez SL (2020) Influence of nitrate supplementation on endurance cyclic sports performance: a systematic review. Nutrients 12(6): 1796.
- Gao C, Gupta S, Adli T, Hou W, Coolsaet R, et al. (2021) The effects of dietary nitrate supplementation on endurance exercise performance and cardiorespiratory measures in healthy adults: a systematic review and meta-analysis. Journal of the International Society of Sports Nutrition 18(1): 55.
- Campos HO, Drummond LR, Rodrigues QT, Machado FSM, Pires W, et al. (2018) Nitrate supplementation improves physical performance specifically in non-athletes during prolonged open-ended tests: A systematic review and meta-analysis. Br J Nutr 119(6): 636-657.
- Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, et al. (2013) Effects of short-term dietary nitrate supplementation on blood pressure, O 2 uptake kinetics, and muscle and cognitive function in older adults. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 304(2): R73-R83.
- Bailey SJ, Vanhatalo A, Winyard PG, Jones AM (2012) The nitrate‐nitrite‐nitric oxide pathway: Its role in human exercise physiology. European Journal of Sport Science 12(4): 309-320.
- Thompson KG, Turner L, Prichard J, Dodd F, Kennedy DO, et al. (2014) Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respiratory Physiology & Neurobiology 193: 11-20.
- Domínguez R, Cuenca E, Muñoz JLM, Fernández PG, Paya NS, et al. (2017) Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients 9(1): 43.
- Arazi H, Eghbali E (2021) Possible effects of beetroot supplementation on physical performance through metabolic, neuroendocrine, and antioxidant mechanisms: A narrative review of the literature. Frontiers in Nutrition 8: 660150.
- Braakhuis AJ, Hopkins WG (2015) Impact of dietary antioxidants on sport performance: A review. Sports Med 45(7): 939-955.
- Jones AM (2014) Influence of dietary nitrate on the physiological determinants of exercise performance: A critical review. Appl Physiol Nutr Metab 39(9): 1019-1028.
- Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, et al. (2013) Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J Appl Physiol 115(3): 325-336.
- Thomas DT, Erdman KA, Burke LM, MacKillop M (2016) Position of the academy of nutrition and dietetics, dietitians of Canada, and the American college of sports medicine: Nutrition and athletic performance. J Acad Nutr Diet 116(3): 501-528.
- Stowe CB (2011) The effects of pomegranate juice consumption on blood pressure and cardiovascular health. Complementary Therapies in Clinical Practice 17(2): 113-115.
- Jurenka JS (2008) Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern Med Rev 13(2): 128-144.
- Trexler ET, Ryan AES, Melvin MN, Roelofs EJ, Wingfield HL (2014) Effects of pomegranate extract on blood flow and running time to exhaustion. Applied Physiology, Nutrition, and Metabolism 39(9): 1038-1042.
- García AT, Gandía VA, Rubia AJL, Ruiz MSA, Calderón MQ, et al. (2019) Pomegranate extract improves maximal performance of trained cyclists after an exhausting endurance trial: A randomised controlled trial. Nutrients 11(4): 721.
- Horiuchi M, Endo J, Dobashi S, Handa Y, Kiuchi M, et al. (2017) Muscle oxygenation profiles between active and inactive muscles with nitrate supplementation under hypoxic exercise. Physiological Reports 5(20): e13475.
- Arefirad T, Seif E, Sepidarkish M, Khonsari NM, Mousavifar SA, et al. (2022) Effect of exercise training on nitric oxide and nitrate/nitrite (NOx) production: A systematic review and meta-analysis. Frontiers in Physiology 13: 953912.
- Martins IJ (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research 5(1): 9-26.
- Martins IJ (2017) Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. Journal of Clinical Epigenetics 3(3): 1-8.
- Martins IJ (2017) Nutrition therapy regulates caffeine metabolism with relevance to NAFLD and induction of type 3 diabetes. 4(1): 1-9.
© 2026 Jestin V Thomas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






