- Submissions

Full Text
Research & Development in Material Science
Chemical Fixation of Carbon Dioxide: Using Material Chemistry to Convert CO2 into Valuable Products
Mohammad Taghi Nazeri*
Department of Organic Chemistry, Shahid Beheshti University, Iran
*Corresponding author:Mohammad Taghi Nazeri, Department of Organic Chemistry, Shahid Beheshti University, Daneshjou Boulevard Street, Tehran, 1983969411, Iran
Submission: July 30, 2025;Published: August 19, 2025

ISSN: 2576-8840 Volume 22 Issue 1
Abstract
Carbon dioxide (CO2) emissions from human activities play a significant role in global warming. The primary methods for reducing CO2 emissions are capturing and storing it or utilizing it. While capture and storage are often not economically justifiable, capturing CO2 from various sources and converting it into valuable products offers economic benefits and contributes to reducing CO2 in the atmosphere. The need for chemical stabilization is becoming increasingly important. CO2 is an inexpensive, non-toxic, renewable carbon source that is both kinetically and thermodynamically stable. To enhance its reactivity, effective catalysts are essential. Therefore, the development of efficient catalysts with unique characteristics and excellent activity and selectivity for various CO2 chemical transformations is crucial. This presentation aims to advance the development of innovative and effective catalysts for reducing greenhouse gases.
Keywords:Carbon dioxide; Catalysts; Cyclic carbonates; Fixation
Introduction
Climate change has become a growing concern worldwide, leading to global warming. The changes stem from greenhouse gas emissions. Carbon dioxide (CO2) is a significant greenhouse gas that is naturally regulated by the carbon cycle, which involves terrestrial plants, oceans, and microorganisms. This cycle has increased dramatically over the past century, primarily due to industrialization and urbanization. It now exceeds 1 trillion tons of CO2 in the atmosphere [1,2]. That is 3.9% of the excess CO2 in the atmosphere, primarily resulting from human activities and the increased consumption of fossil fuels [3]. It is estimated that by 2100, with continued increases in CO2 emissions, the Earth’s temperature will rise by 5.2 degrees, resulting in increased sea level rise and ocean acidification. These climate change issues pose a serious threat to human life [4]. The question naturally arises as to how to actively remove CO2 from the atmosphere, not just as a waste product, but as a strategic raw material for the manufacture of valuable materials, which poses a significant challenge for the 21st century [5]. Extensive research is underway to reduce CO2 in the atmosphere. It is based on three general approaches. The first approach is to reduce CO2 production, which can be achieved by replacing fossil fuels with clean and renewable energy carriers [6]. The second approach is to store CO2, which can be achieved by capturing and separating CO2 through the development of new and emerging technologies. Despite its potential, the attempt toward effective CO2 capture is hindered by numerous challenges, particularly in terms of costs and securing public support. The third approach is to intelligently use CO2 as a chemical feedstock for the manufacture of functional materials. In the meantime, the chemical conversion of CO2 into valuable products has become an exciting area of research for chemists and materials scientists. These CO2 conversions can be divided into seven general categories, including chemical, electrochemical, photochemical, thermochemical, biological, reforming, and inorganic conversions (Figure 1), with extensive studies being conducted in each section [7].
Figure 1:General CO2 transformations.

Figure 2:Various chemicals are produced from CO2.
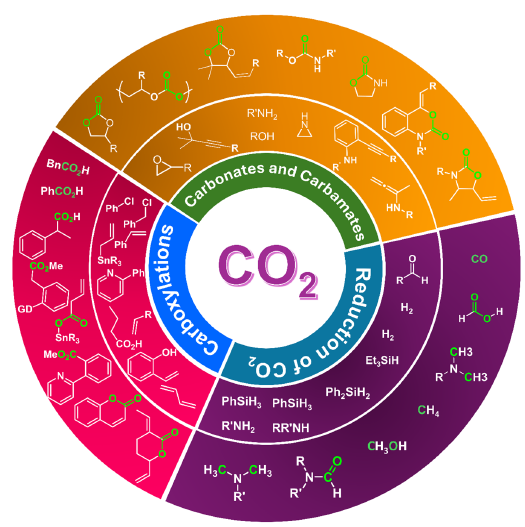
CO2 is a renewable, inexpensive, non-toxic, and abundant carbon source in nature. CO2 is a valuable and recyclable singlecarbon (C1) building block in organic synthesis [8]. Therefore, the chemical fixation of CO2 is one of the most vital topics in the academic community and industry. In recent years, considerable efforts have been devoted to this specific topic. Organic chemists have developed various methods to convert CO2 into valuable chemicals. For instance, they have extensively studied the synthesis of carbonates and cyclic polycarbonates using CO2 and epoxides, as well as carboxylation reactions involving CO2, the reduction of CO2, and many other promising reactions (Figure 2); [9]. The conversion of CO2, considered an industrial waste gas, into beneficial compounds is of significant importance. Products created through CO2 fixation hold considerable economic value. Among these products, the reaction of CO2 with epoxides to form cyclic carbonates has gained significant attention due to its 100% atom economy [10]. Cyclic carbonates, the desired end products, are widely used as polar aprotic solvents in chemical processes, as electrolytes in lithium-ion batteries, and as intermediates in the production of fine chemicals. CO2 represents the most oxidized form of carbon, making it thermodynamically stable and kinetically inert in certain desired transformations [11]. Accordingly, it requires a highly reactive substrate or harsh reaction conditions to activate CO2, but such conditions have limitations. Therefore, there is a need for a material that can adsorb and activate the substrate and CO2, while also facilitating the coupling of CO2 to energy-rich substrates, such as epoxides and aziridines, to produce polycarbonates/ polycarbamates and/or cyclic carbonates/carbamates [5,7]. For this purpose, both reactions and catalysts need to be developed. A large number of inorganic, organic, and metallic catalysts have been developed for various chemical transformations of CO2.
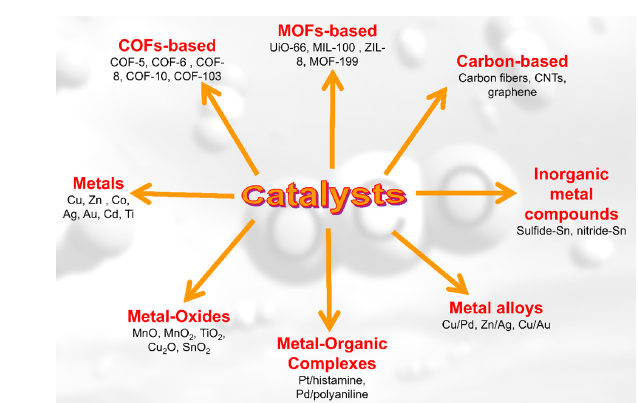
Living organisms have evolved to exist in a remarkably diverse range of environments and can extract energy from their surroundings, converting it into usable energy. This is an exciting model that can benefit catalysis researchers, as it provides a deep understanding of these vital metabolic processes [1]. Catalysis scientists examine the green chemical transformations that natural biological systems can create, which can inspire the development of artificial catalysts. CO2 fixation catalysts are defined by their capability to adsorb and activate CO2 and its reactants, facilitating the chemical conversion of CO2 into valuable products under specific reaction conditions [2]. Important properties of these catalysts include their effectiveness in adsorbing and activating CO2 and reactants, as well as selectivity, stability, recyclability, and considerations of cost-effectiveness and sustainability. In recent years, various catalysts have been developed for CO2 fixation in diverse applications. They can be broadly classified into two categories: homogeneous and heterogeneous catalysts [12]. Homogeneous catalysts, particularly those that are metalbased, play a crucial role in various chemical reactions and include catalysts containing transition metals such as copper (Cu), cobalt (Co), ruthenium (Ru), silver (Ag), nickel (Ni), palladium (Pd), gold (Au) and tungsten (W) [13]. These catalysts are renowned for their exceptional catalytic activity and selectivity, enabling them to facilitate reactions with high efficiency and precision. Their ability to offer specific pathways for reactants to convert into products makes them valuable in fine chemical synthesis and industrial applications. Despite the advantages offered by homogeneous catalysts, heterogeneous catalysts tend to be more prevalent in practical applications. This preference is largely attributed to their ease of handling and the ability to separate and recycle them from reaction mixtures easily. Additionally, heterogeneous catalysts often demonstrate robust stability and can operate effectively under a wider range of conditions, including mild temperatures and pressures, making them suitable for large-scale industrial processes [14]. This combination of factors contributes to their widespread use, as they provide both economic and operational benefits in various chemical processes. Recent advances in catalytic systems have led to the development of various heterogeneous catalysts for the chemical fixation of CO2. Among these catalysts are natural polymers such as cellulose, chitosan, pectin, etc., which demonstrate excellent capabilities in catalyzing CO2 fixation due to their unique structures [8]. For instance, Sun et al. [15] reported for the first time an efficient, reusable, and environmentally friendly catalytic system made from sugarcane bagasse and potassium iodide (KI). This system effectively catalyzes the cyclization of CO2 into epoxides or aziridines under mild conditions, successfully enabling the synthesis of cyclic carbonates. To enhance the catalytic performance of natural polymers in CO2 fixation, they are often functionalized with additional materials. This functionalization improves their catalytic properties and chemical stability. By specifically targeting the functionalization of natural polymers, a synergistic effect is created between the existing hydroxyl groups and the created functional group. This interaction enables the simultaneous activation of reactants and CO2, which, in turn, increases the catalytic activity of the natural polymers. Jia and co-workers successfully covalently attached a non-metallic complex synthesized from salicylaldehyde and an amine compound to chlorinated cellulose through a multicomponent reaction. The presence of hydroxyl, phenolic -OH, and Lewis base (imine) functional groups in this catalyst contributes to its excellent activity in CO2 fixation [16]. In another instance, a metal is utilized to improve the catalytic activity of natural polymers [17,18]. For example, Nazeri et al. [19] covalently bonded various natural polymers with metal-containing phthalocyanines through a multi-component reaction. They employed these materials in the CO2 fixation reaction, achieving outstanding catalytic performance [19,20]. Metal-organic frameworks (MOFs) are effective materials for capturing and converting CO2 due to their high surface area, porosity, and adjustable properties. Characterizing MOFs for CO2 fixation requires an understanding of their structural, textural, and chemical properties, as well as their interactions with CO2 [20,21]. Kim et al. [21] created bifunctional pyridinium ionic MOF catalysts by N-alkylating pyridine sites in porous MOFs. The combination of acidic sites and nucleophilic anions significantly improved catalytic activity for cyclizing CO2 with epoxides to form cyclic carbonates under mild, solvent-free conditions. In recent years, metal-free amorphous porous organic polymers (POPs) and crystalline covalent organic frameworks (COFs) have demonstrated their extraordinary potential as solid-state organic catalysts forCO2 fixation reactions, owing to their high intrinsic porosity and structural stability, which support active functional groups [22]. Mesoporous silica has a highly ordered hexagonal structure characterized by uniform mesopores. This material is notable for its large surface area, adjustable pore size, and stability in both thermal and mechanical contexts. Due to these properties, it serves as an effective adsorbent and activator in the CO2 fixation process. Accordingly, Nazeri and colleagues converted SBA-15 into modified SBA-15-NH. Through a multi-component reaction, SBA-15-NH was covalently attached to cobalt phthalocyanines, which were able to fix CO2 into a cyclic carbonate with a high capacity to absorb and activate CO2 in the shortest time [23]. Another type of carbon-based catalyst, which includes graphene, carbon nanotubes, and carbon nanofibers, is widely used in CO2 fixation due to its properties, such as high surface area, tunable porosity, good electrical conductivity, chemical and mechanical stability, and resistance to harsh conditions [24]. Nazeri et al. [25] designed a targeted strategy based on the coupling of multi-walled carbon nanotubes (MWCNTs) with tetra-amino cobalt phthalocyanine (Co-PcTA) via an efficient four-component reaction, the Ugi (Ugi-4CR). The prepared catalyst exhibits a high CO2 absorption capacity and high efficiency in the chemical conversion to cyclic carbonate (Figure 3).
Figure 3:
This report addresses the unusual increase in CO2 and its dangers. Organic chemists have extensively studied the chemistry of CO2 and have introduced this unique and straightforward compound as an interesting precursor for conversion into a variety of valuable and functional products. This stable and inert material requires a catalyst for the reaction to occur. Various catalysts have been identified for use in CO2 fixation. It is hoped that the study of these catalysts will lead to the development of more efficient and industrially viable catalysts to address the world’s CO2 problem.
Conclusion and Outlook
The chemical fixation of CO2 and its conversion into beneficial compounds is a primary objective for organic chemists. CO2 is a compound that exhibits both kinetic and thermodynamic stability. Catalysts are crucial for enhancing their reactivity, and in recent years, numerous new catalysts have been developed. This study examines various types of catalysts employed in the chemical conversion of CO2 into valuable materials. Currently, the recovery of CO2 and its removal from the atmosphere, along with its transformation into useful substances, have become practical technologies. Although many publications have emerged in this field, substantial research is still needed to establish CO2 as a raw material for CO2 reduction technologies. Therefore, research efforts should focus on developing rapid and economically viable methods for the fixation and utilization of CO2. We hope that the ongoing research and publications will lay a solid foundation for further advancements in this area.
Acknowledgment
This paper has been supported by the Research Council of Shahid Beheshti University.
References
- Appel AM, Bercaw JE, Bocarsly AB, Dobbek H, DuBois DL, et al. (2013) Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 Chem Rev 113: 6621-6658.
- Bierbaumer S, Nattermann M, Schulz L, Zschoche R, Erb TJ, et al. (2023) Enzymatic conversion of CO2: from natural to artificial utilization. Chem Rev 123: 5702-5754.
- Balat H, Öz C (2007) Technical and economic aspects of carbon capture a storage-A review. Energy Explor Exploit 25: 357-392.
- Aresta M, Dibenedetto A (2007) Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans, pp. 2975-2992.
- He M, Fang Z, Wang P, You Y, Li Z (2023) Recent progress in photocatalytic chemical fixation of carbon dioxide. ACS Sustain Chem Eng 11: 12194-12217.
- Wang S, Zhang L, Wang P, Liu X, Chen Y, et al. (2022) Highly effective conversion of CO2 into light olefins abundant in ethene. Chem 8(5): 1376-1394.
- Mikkelsen M, Jørgensen M, Krebs FC (2010) The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ Sci 3: 43-81.
- Claver C, Yeamin MB, Reguero M, Masdeu-Bultó AM (2020) Recent advances in the use of catalysts based on natural products for the conversion of CO2 into cyclic carbonates. Green Chem 22: 7665-7706.
- Maeda C, Miyazaki Y, Ema T (2014) Recent progress in catalytic conversions of carbon dioxide. Catal Sci Technol 4: 1482-1497.
- Alves M, Grignard B, Méreau R, Jerome C, Tassaing T, et al. (2017) Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: catalyst design and mechanistic studies. Catal Sci Technol 7: 2651-2684.
- Usman M, Rehman A, Saleem F, Abbas A, Eze VC, et al. (2023) Synthesis of cyclic carbonates from CO2 cycloaddition to bio-based epoxides and glycerol: an overview of recent development. RSC Adv 13: 22717-22743.
- Rajanna D, Srinivasappa PM, Sudhakaran A, Biradar A, Samal AK, et al. (2024) Recent advances in the fixation of CO2 into quinazoline and benzimidazole. Energy & Fuels 38: 8481-8515.
- Frontera P, Macario A, Ferraro M, Antonucci P (2015) Catalysts, p. 59.
- Javanbakht S, Nasiriani T, Farhid H, Nazeri MT, Shaabani A (2022) Sustainable functionalization and modification of materials via multicomponent reactions in water. Front Chem Sci Eng 16: 1318-1344.
- Chen W, Zhong LX, Peng XW, Sun RC, Lu FC (2015) Chemical fixation of carbon dioxide using a green and efficient catalytic system based on sugarcane bagasse-An agricultural waste. ACS Sustain Chem Eng 3: 147-152.
- Chen S, Pudukudy M, Yue Z, Zhang H, Zhi Y, et al. (2019) Nonmetal schiff-base complex-anchored cellulose as a novel and reusable catalyst for the solvent-free ring-opening addition of CO2 with epoxides. Ind Eng Chem Res 58: 17255-17265.
- Nazeri MT, Ghasemi M, Torabi S, Shaabani A (2024) Functionalization of poplar cotton via azido-Ugi reaction: as a green biocatalytic system for the chemical fixation of CO2. Cellulose 31: 4929-4946.
- Hu L, Xie Q, Tang J, Pan C, Yu G, et al. (2021) Co (III)-Salen immobilized cellulose nanocrystals for efficient catalytic CO2 fixation into cyclic carbonates under mild conditions. Carbohydr Polym 256: 117558.
- Nazeri MT, Javanbakht S, Nabi M, Shaabani A (2022) Copper phthalocyanine-conjugated pectin via the Ugi four-component reaction: An efficient catalyst for CO2 Carbohydr Polym 283: 119144.
- Nazeri MT, Ramezani M, Javanbakht S, Shaabani A (2022) Chemical CO2 fixation using a green biocatalytic system based on Ugi conjugated cobalt phthalocyanine on cellulose. Sustain Energy Fuels 6: 5134-5145.
- Ji H, Naveen K, Lee W, Kim TS, Kim D, Cho DH (2020) Pyridinium-functionalized ionic metal–organic frameworks designed as bifunctional catalysts for CO2 fixation into cyclic carbonates. ACS Appl Mater Int 12: 24868-24876.
- Modak A, Ghosh A, Mankar AR, Pandey A, Selvaraj M, et al. (2021) Cross-linked porous polymers as heterogeneous organ catalysts for task-specific applications in biomass transformations, CO2 fixation, and asymmetric reactions. ACS Sustain Chem Eng 9: 12431-12460.
- Nasiriani T, Nazeri MT, Shaabani A (2023) Cobalt phthalocyanine conjugated SBA-15 mesoporous silica via the Ugi four-component reaction: A potential heterogeneous catalytic nanocomposite for CO2 fixation reaction. Microporous Mesoporous Mater 354: 112514.
- Zhang Z, Gao H, Wu H, Qian Y, Chen L, et al. (2018) Chemical fixation of CO2 by using carbon material-grafted N-heterocyclic carbene silver and copper complexes. ACS Applied Nano Materials 1: 6463-6476.
- Nazeri MT, Javanbakht S, Ramezani M, Shaabani A (2022) A facile and green synthesis of cobalt phthalocyanine-conjugated multiwall carbon nanotube by the Ugi reaction: as an efficient CO2 fixation catalyst. J Taiwan Inst Chem Eng 136: 104428.
© 2025 © Mohammad Taghi Nazeri. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






