- Submissions

Full Text
Researches in Arthritis & Bone Study
The Westergren Sedimentation Rate: is Automation Undermining Clinical Accuracy?
John Abner Goldman1*, Goldman John Michael2 and Parris Glenn R3
1Clinical Professor of Medicine, Rheumatology, Immunology, Emeritus, Emory School of Medicine, Georgia
1MAA, CPA, Peachtree Orthopedics, Georgia
1Northside Hospital, Gwinnett, Parris and Associates, Georgia
*Corresponding author:John Abner Goldman, Clinical Professor of Medicine, Rheumatology, Immunology, Emeritus, Emory School of Medicine, Emory Saint Joseph’s Hospital, Northside Hospital and Parris and Associates, Atlanta, Georgia
Submission: December 01, 2025;Published: December 17, 2025
Volume2 Issue2December 17, 2025
Introduction
The Westergren Sedimentation (WSR) is a valuable, inexpensive laboratory test to guide physicians and rheumatologists in diagnosis and treatment that correlates with systemic inflammation. [1,2] The test is used to guide the diagnosis, monitoring, and treatment of inflammatory disease. [1,2] It Evaluates Red Cells in Eidetic Acid (EDTA) anticoagulated blood to fall in one hour in a vertical column of blood in a tube. The tubes are either plastic or glass, internal diameter of 2.5mm, lengths of 190 to 300mm. 3 tube coefficient of variation were performed in 15 patients. This investigation was initiated to see the present status of the erythrocyte sedimentation rate and its availability for practicing physicians.
Patients and Methods
The in-office testing used the manual Laboratory Sedimentation Rate test, but the central laboratory uses an Automated system called iSED® by Alcor, which directly measures RBC aggregation with capillary photometry during rouleaux formation (CPSR). These were performed on 106 de-identified patients with dual specimens; one obtained in the office and the other sent to the central laboratory. Patients included various outpatient populations across multiple rheumatology diagnoses. Statistical analysis included Passing-Bablok regression and paired T-tests, chosen for their robustness in comparing methods and identifying systematic differences between the two testing’s. We did not require an institutional review board or ethics committee approval.
Background
The first to notice a change in blood sedimentation due to illness was a British surgeon John Hunter (1728-1793), in his posthumous publication, a treatise on the blood, inflammation and gun-shot wounds [3,4]. A Polish physician, Edmund Faustyn Biernacki (1866-1911), later refined the clinical use of the Erythrocyte Sedimentation Rate (ESR) near the end of the 19th century [5]. Biernacki detailed his findings in two articles in 1897 (the Gazeta Lekarska in Poland and the Deutsche Medizinische Wochenschrift in Germany) and developed his test for measurements. These findings were not widely propagated in the English-speaking medical communities. Because of his work, the ESR is occasionally called the Biernacki Reaction worldwide [6]. The applied use of ESR in clinical diagnostics by Biernacki was refined by Dr. Robin Fahraeus in 1918 and Dr. Alf Vilhelm Albertsson Westergren in 1921 [7,8]. Dr. Westergren defined the standard measurement of the ESR that is still in use today [7].
Together, Robin Fahraeus and Alf Vilhelm Albertsson Westergren are often remembered for the test, historically called the Fahraeus- Westergren test (FW test or Westergren test), which uses standardized vertical tube and sodium citrate anticoagulated [now eidetic acid (EDTA)] anticoagulated blood [7,8]. They described the WSR result as the “sinking velocity”.
Despite being over a century old, the Westergren Sedimentation Rate (WSR) remains a vital laboratory marker, particularly in assessing inflammatory activity in autoimmune and infectious diseases. However, as clinical laboratories increasingly adopt automated analysers such as the CPSR-now used in a growing number of hospital and outpatient settings worldwide-concerns have arisen regarding discrepancies in results compared to traditional WSR. This retrospective study of 106 paired samples compared WSR and the capillary photometry sedimentation rate results and revealed statistically significant differences in measurements. These findings raise concerns that replacing WSR with automated methods could mislead clinical decisions, especially in rheumatology. Given the persistent relevance and costeffectiveness of WSR, further research and caution are warranted before fully retiring this traditional method. It also noted that doing a fresh WSR in the office avoids the time for delivery to a Central Lab (CRL). Handling and transportation-sometimes up to 24 hours before testing-can impact WSR results.
A WSR must be done rapidly while the specimen is fresh (the usual time frame is less than 2 hours) but we say STAT. But many Americans are on insurance programs that use a Central Reference Laboratory (CRL). Many laboratory studies can be done in the Physician’s Office Labs (POL) but often, insurance dictates that all labs are sent to specified CRL. This study was done to examine the wisdom of demanding the WSR be sent to CRL and to evaluate how CRLs are performing an Automated Sedimentation Rate (ASR). We want to know how it compares with the concordance and accuracy of the WSR versus the commercial Erythrocyte Sedimentation Rate (ESR) sent to CRL. The methodology of the CRL is now automating large numbers of specimens via photo cytometry of the ESR and how it compares to the manual WSR. They are measuring RBC aggregation with capillary photometry during rouleaux formation. The erythrocytes are negatively charged and impacted by positively charged large proteins which are acute phase proteins like fibrinogen and immunoglobulins [3-5]. Keeping this in mind, we tested 106 de-identified patients on managed care programs that required all labs to be sent to the CRL and not the POL. There were 94 on Cigna, 2 on Atena, and 10 on other programs.
Result
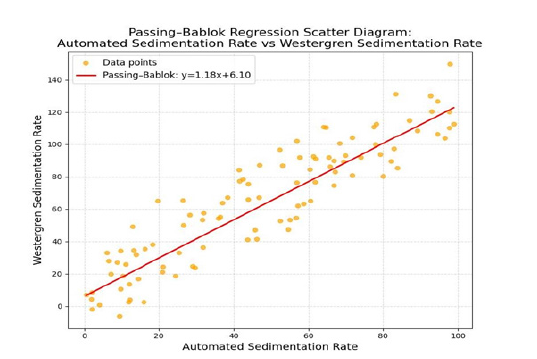
The information on the disease comparisons is with the Passing Bablok regression analysis scatter graph.
These were the diagnoses of the 106 patients whom we tested. (Table 1 & 2) (Figure 1).
Table 1:These were the diagnoses of the 106 patients tested.
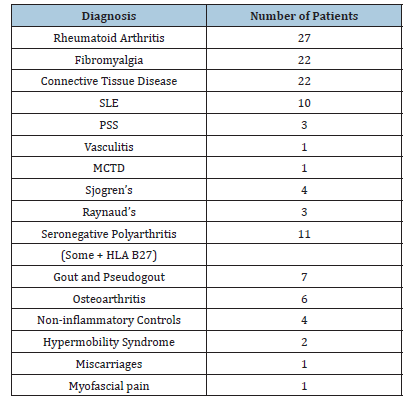
Table 2:Grouping of the western sedimentation rate results and statistical evaluation using paired T-testing.
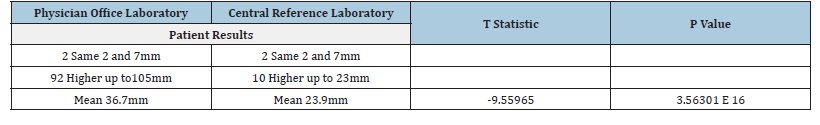
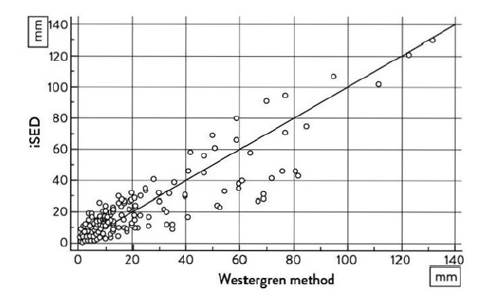
Figure 1:Data from the study of the Passing-Bablok regression analysis scatter diagram comparing the manual Westergren Sedimentation Rate (WSR) done in the office laboratory versus the send-out specimen performed at the Central Laboratory (CRL) using the automated sedimentation rate.
Perspectives on statistics and data
Since there is deviation, this recalls the refrain allegedly developed by Lord Beaconsfield or often attributed to Benjamin Disraeli (1804-1881) who was Prime Minster of Great Britain from 1874 to 1880. Because of this Mark Twain ascribed it to him in a 1907 article in the North American Review: “Figures often beguile me...particularly when I have the arranging of them myself; in which case the remark attributed to Disraeli and would often apply with justice and force: “There are three kinds of lies: lies, damned lies, statistics [9].”
Internal evaluation some of the readings are not on the regression analysis straight line. These are outliers. It is not a straight line with the actual data.
Alcor iSED data
Figure 2:Coefficient of Variation as seen in 18 of our patients. The coefficient of variation in 18 patients. We see ups and downs. There is less variation with high sedimentation rates as compared to lower sedimentation rates. Ideally, the result should be less than 15%. Compared to the Westergren method, the CPSR requires >96% less hands-on time and reduces turnaround time up to 94%.
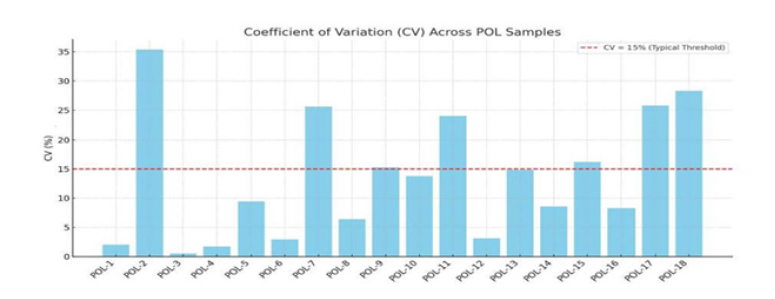
The 3-tube Coefficient Variation (CV) depends on the value of the sedimentation rate of 1.7 % to 2.94% in the higher numbers to as high as 13.85% to even 25.61 % with low numbers [2]. This parallels our experience. This is expected because it is inversely related to the mean, and if the mean increases, the CV decreases even if the standard deviation remains constant. This characteristic makes the CV particularly useful for comparing the relative variability of data sets with different units or scales. The Alcor iSED describes similar findings but has not published its numbers. The other authors we have reviewed do not publish it either. We have published actual numbers in this manuscript. With experience, the CV numbers improve, but there is a deviation (Figure 2). The deviation from the mean and reference range are cited by the CRL for the ESR. This is often set at the upper limit of normal, above 30mm per hour whereas the POL WSR cut-off is usually 20mm per hour.
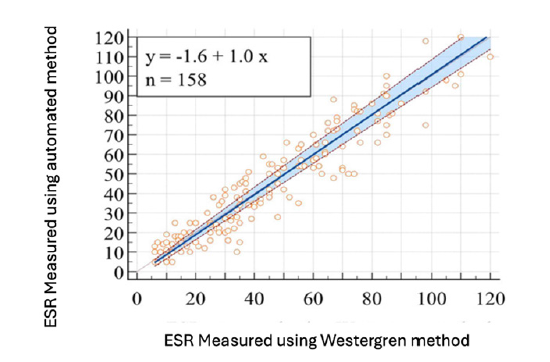
Sox and Liang and Miller suggest that normal results are better stated for women as ½ (their age plus 10), while for men it is ½ their age [10,11]. This normal result is what we do. It is uncertain whether this works for the capillary photometry sedimentation rate results, but it should. CPSR brings a novel approach to the ESR by directly measuring RBC aggregation with capillary photometry during rouleaux formation. Results are available in seconds, and the CRL indicates they are highly correlated to the Westergren method by general statistics but may not be usable by the clinical rheumatologist. This is statistics again. They have almost no disposables or sample setup required. Capped primary EDTA tubes can be directly inserted into the instrument, and mixing, sampling, and reading are performed by the capillary photometry sedimentation rate results for true walk-away automation (Figure 3) [12-15]. Passing Bablok scatter diagram for iSED® results of Čičak H, Šonjić P, Šimundić AM [16].
Figure 3:Čičak H, Šonjić P, Šimundić AM. Verification of automatic analysers Roller 20PN and iSED for measuring erythrocyte sedimentation rate [15]. Both analysers, Roller 20PN and iSED, are not comparable with the Westergren method and should not be used interchangeably. Overall, the disagreement with the Westergren method is less pronounced for the iSED analyzer. As we notice, there are some outliers beyond the equivalent line, which therefore makes us concerned about the result.
Precision [11-15] - indicates
Samples spanning a range of ESR values (quartiles were defined as per the CLSI ESR standard) were run on the iSED® ELITE analyzer a total of ten [10] times in the same to demonstrate precision. This is in the same laboratory and not transported as it is for physicians’ offices. As with other laboratory tests, they point out, higher CVs are expected when comparing lower numerical values. They have not published their numbers yet, but it appears it’s the same as we have seen. There are other automated systems available. We have asked Elkhorn to update their Passing-Bablok information on more than once but we have not received it.
Data Collection
The timing of processing of a CRL run ESR may be anywhere from a couple of hours to as many as 24 hours, depending on the pickup time and the runtime (personal communication). This time interval should be cited on the lab report when reporting the result to physicians. In the past, it had been printed and still is by Quest Diagnostics Incorporated, but not by LabCorp. The consequences of inaccurate reporting of WSR can relate to medical decisionmaking. The WSR is used clinically by itself and in the DAS-28ESR for Rheumatoid Arthritis [16].
How much of a difference may affect clinical decisions is uncertain.
Discussion
This investigation was initiated to see the status of the erythrocyte sedimentation rate and its availability for the practicing physician. A WSR must be done rapidly while the specimen is fresh (the usual time frame is 2 hours), but we say STAT in the office. Many Americans are on insurance programs that require a central laboratory or allow a POL that requires CLIA certification, which requires paperwork, inspection and costs. Since laboratory studies can be done in the Physician’s Office Labs (POL), but sometimes insurance demands that all labs be performed in the CRL. It is wise that in the office, the WSR by CLIA is a CLIA-waved test [17]. This test requires a CLIA certificate or a CLIA-waived certificate for payment. On the CLIA website It is stated as ERYTHROCYTE SEDIMENTATION RATE, NONAUTOMATED, WAIVED. We performed this study because we have seen results where if there is a delay in running the WSR then the results are lower. Different authors have said it must be performed within 30 minutes to 4 hours after it is drawn at room temperature and 24 hours if stored in a refrigerator [18-27].
The dilemma is the total time it takes to be transported to the CRL and to perform it after it is drawn. Some of the CRL are miles away from the physician’s office, including in different states and the blood tubes are delivered by courier, or are flown to these different sites. Alternatively, the labs are drawn up in the office all day and delivered to the transportation site in the evening. All of this may take up to 24 hours or more. This may not be crucial for other laboratory tests, but it is crucial for the sedimentation rate. Depending on why the WSR is elevated or low can affect this. We feel it is an important reason for the outcome. Since this is a different technique, does this still apply? It should. Different clinical diagnoses can affect the WSR. Is this the same as the CPSR? Dhall and Taneja confirmed earlier works that the WSR goes up in pregnancy and goes up to the 7th day in the puerperium before going down [18]. This needs to be included in an assessment of a pregnant patient. It has not been validated by the CRL for the CPSR but it should. Čičak H, Šonjić P, Šimundić AM noted both analysers, Roller 20PN and iSED, are not comparable with the Westergren method and should not be used interchangeably. Overall, the disagreement with Westergren method is less pronounced for iSED analyser [15].
Zhbanov and Yang have looked at electricity as an important mechanism to evaluate sedimentation [19]. Red blood cells have a negative charge. They experimentally demonstrated that the disaggregated cells settle much slower than the aggregated cells which supports the Rouleaux phenomenon. They had not looked at the delayed running of the WSR. The CPSR has not yet been evaluated for the delayed running of the WSR. These are the areas in the future research we need since CPSR is a different technology. This is important because we need to have faith in disease tracking with WSR and the DAS28ESR [16]. As compared with the CRP, the WSR is a more useful laboratory parameter in assessing Polymyalgia rheumatica/Giant cell arteritis (PMR/GCA) [20]. Comparing the DAS28ESR and DAS28CRP, the American College of Rheumatology (ACR) recommendations for use in clinical Practice has stated that these numerical results are the same though many articles have questioned this [16,20].
Figure 4:Plot of passing-bablok regression analysis from walle paper [28]. This is another automated sedimentation rate SIFR ESR 300.
We need to know that the DAS28ESR score for remission is <2.6, but the DAS28CRP score for remission is about <2.3. These numbers are not the same. Sengul noted that there is a discordance of DAS28ESR and DAS28CRP when the validated thresholds for DAS28ESR are being used for DAS28CRP [21]. This led to errors in the determination of disease activity, which can lead to errors in the management of patients with rheumatoid arthritis [22]. has noted that WSR testing should be done within 4 hours of sample collection [22]. Yardumian also supports the 2-hour limit [23]. Xiaoyang and Chenming also support the 2-hour criteria [24]. Tishkowski and Gupta have agreed the test should be done by 2 hours and that mechanical issues need to be evaluated, including those that can increase WSR and this includes proteins in the acute phase, such as prothrombin, plasminogen, fibrinogen, C-reactive protein, alpha 1 antitrypsin, haptoglobin and complement proteins [25]. Narang felt that the automated testing can safely replace the WSR except for high ESR values which they advise validation by the manual WSR method [26]. How would you do that without a delay to discover if there is a high level? Jeremia recommended the WSR be conducted immediately since doing it then is more effective than delaying it [27]. Walle et al felt that comparing the SIFR ESR 300, another automated test, with the manual WSR showed a strong correlation between them and a good agreement [28] (Figure 4).
Limitations
We see there are various issues and factors that can affect the WSR. These can be physical, immune-related, chemical, electrical, timing, criteria, tube size and technique. The practicing physician needs to be aware of these when evaluating the patient. As rheumatologists, we need to be aware of these as they are crucial to care. The various authors we have reviewed say that the WSR should be run within 2 hours. According to CRL they are unable to reach that goal because of delivery issues. Limitations of this study include its retrospective design, which may introduce selection and information biases. Additionally, the lack of disease-specific subgrouping could obscure condition-dependent variability in ESR values, and the limited sample size may affect the generalizability of the results. These limitations could potentially influence the observed level of discordance between the WSR and CPSR methods, underscoring the need for cautious interpretation and further prospective studies. Also, lack of disease-specific subgrouping, and limited sample size may play a role. Nonetheless, the findings are compelling enough to warrant caution in replacing WSR entirely with automated methods, especially in specialties where ESR plays a critical clinical role.
Koepke has welcomed the innovation in erythrocyte sedimentation rate [29]. Although he wrote his article in 2002, even then he was interested in the changes in the evaluation of the sedimentation rate in shorter time intervals. He wrote, “Clinical laboratories are facing increased workloads with decreasing numbers of well-trained personnel and shrinking budgets. By using innovative technologies, laboratories can better cope with these changes. We look for continuing improvements in many other aspects of laboratory medicine.” This was true in 2002 and still is now in 2025.
Summary and Conclusion
Significance & innovation
This investigation was initiated to see the status of the erythrocyte sedimentation rate and its availability for the practicing physician. We see there are various issues and factors that can affect the WSR. These can be physical, immune related, chemical, electrical, timing, criteria, tube size and technique. The practicing physician needs to be aware of these when evaluating the patient. As rheumatologists, we need to be aware of these as they are crucial to care. While we performed the Western sedimentation rate in the office, some of our patients who had received their lab at a central laboratory where an outcome of the sedimentation rate that was lower than what we saw in the office. This initiated our interest in comparing the sedimentation rate we do in the office with the one that was sent to the Central Laboratory. The study presented a comprehensive analysis of patient data through detailed statistical methodologies and graphical representations. These include correlations drawn from diagnostic groups and variability assessment, aimed at enhancing the understanding of clinical outcomes and methodological reliability. The study presented a comprehensive analysis of patient data through detailed statistical methodologies and graphical representations. These include correlations drawn from diagnostic groups and variability assessment, aimed at enhancing the understanding of clinical outcomes and methodological reliability.
When we send a blood specimen to an outside laboratory, we need to remember that this is not a comparison, as you perform all tests done in the same room or building. The specimen that is sent out and gets shaken a lot, and this can lower a WSR [30]. Is this the same for an automated test? We don’t know but maybe one of the many reasons is that the CRL ESR result is lower. Our data was done at the POL and an additional specimen was sent to the CRL, but the other data in the literature was done in the same laboratory or at least the same building. This may be another reason for the outliers. We have reported this at our annual meeting.
These are the conclusions which we have ascertained.
a. The WSR is a simple but important test. It is non-specific.
b. It needs to be run as soon as possible after drawing.
c. It would be best run in the physician’s office if the
physician can afford to run the WSR.
d. Employed physicians may not have a choice.
e. Fortunately, it is a CLIA-waived test that can be run and
paid for by insurance and federal programs with and without a
full CLIA certificate.
f. It is a better test to evaluate the patient when it is
performed in the POL.
g. The DAS28ESR depends on it and is better than the
DAS28CRP. It is important that the American College of
Rheumatology clarifies that the same numerical criteria that
are being used for both should not be used for both! [11,22].
Remission by the DAS28ESR is <2.6, but not for the DAS28CRP,
since it is closer to <2.3 for remission. This needs correction.
h. Statistics can be a problem when developing the Passing-
Bablok regression analysis.
i. The CPSR may have some advantages, but the WSR run in
the POL is better for patient care.
References
- (1993) ICSH recommendations for measurement of erythrocyte sedimentation rate. International council for standardization in haematology (expert panel on blood rheology). J Clin Pathol 46(3): 198-203.
- Walter JF (1945) Mechanism and clinical significance of the erythrocyte sedimentation rate. MD Thesis 1348:
- Michele C, Gloria A, Valentina F, Roberta R, Fabio B, et al. (2024) Method comparison of erythrocyte sedimentation rate automated systems, the VES-MATIC 5 (DIESSE) and Test 1 (ALIFAX), with the reference method in routine practice. J Clin Med 13(3): 847.
- Madrenas J, Potter P, Cairns E (2005) Giving credit where credit is due: John hunter and the discovery of erythrocyte sedimentation rate. Lancet 366(9503): 2140-2141.
- Grzybowski A, Sak J (2011) Edmund biernacki (1866-1911): Discoverer of the erythrocyte sedimentation rate. On the 100th anniversary of his death. Clin Dermatol 29(6): 697-703.
- Tishkowski K, Zubair M (2025) Erythrocyte sedimentation rate. Stat Pearls Publishing, Treasure Island, Florida.
- Fåhraeus R (1929) The suspension stability of the blood. Physiological Reviews 9(2): 241-274.
- Bray C, Bell LN, Liang H, Farah K, Joseph JM, et al. (2016) Erythrocyte sedimentation rate and c-reactive protein measurements and their relevance in clinical medicine. WMJ 115(6): 317-321.
- Twain M (1907) "Lies, damned lies, and statistics," My Autobiography, published in the North American Review in 1907.
- Miller A, Green M, Robinson D (1983) Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J (Clin Res Ed) 286(6361): 266.
- Sox HC, Liang MH (1986) The erythrocyte sedimentation rate. Guidelines for rational use. Ann Intern Med 104(4): 515-523.
- https://alcorscientific.com/clinical-lab/ised-elite-us-esr-analyzer/
- Nihal B, Yilmaz FM, Sezer S, Oğuz E (2015) Comparison of iSED and ves-matic cube 200 erythrocyte sedimentation rate measurements with westergren method. J Clin Lab Anal 29(5): 397-404.
- Patel S, Jhala D (2019) Advantages of the use of alcor iSED automated sed rate analyser vs manual sed rate in evaluation and/or monitoring the treatment in patients: CMCVAMC experience. Am J Clin Pathol 152(Supp-1): S85-S91.
- Čičak H, Šonjić P, Šimundić AM (2022) Verification of automatic analysers roller 20PN and iSED for measuring erythrocyte sedimentation rate. Biochem Med (Zagreb) 32(1): 010708.
- Jaclyn KA, Lani Z, Liron C, Kaleb M (2011) Measures of rheumatoid arthritis activity. Arthritis Care Res 63(Supp-11): S14-S36.
- https://seed.nih.gov/sites/default/files/2024-12/CLIA-Waived-Tests.pdf
- Dhall SH, Taneja OP (1956) Study of erythrocyte sedimentation rate changes in obstetrics and gynaecology. Journal of Obstetrics and Gynaecology of India pp. 435-446.
- Zhbanov A, Yang S (2015) Effects of aggregation on blood sedimentation and conductivity. PLOS One 10(6): e0129337.
- Kyle V, Cawston TE, Hazelman BL (1989) Erythrocyte sedimentation rate and C reactive protein in the assessment of polymyalgia rheumatica/giant cell arteritis on presentation and during follow-up. Ann Rheum Dis 48(8): 667-671.
- Sengul I, Ince B, Seniz AY, Kaya T, Altinay GK (2015) Comparison of the DAS28-CRP and DAS28-ESR in patients with rheumatoid arthritis. Int J Rheum Dis 18(6): 640-645.
- Hu QL, Lin L, Zhang L, Wu LF, Chen MY, et al. (2022) Effect of storage temperature and time on erythrocyte sedimentation rate. Eur J Med Res 27(1): 76-81.
- Yardumian K (1937) Physicochemical factors influencing the red cell sedimentation rate. American Journal of Clinical Pathology 7(2): 105-119.
- Xiaoyang Y, Chenming X (2013) The influence of time and temperature placing the samples on the erythrocyte sedimentation rate (WSR). Guangzhou Medicine 44: 50-51.
- Tishkowski K, Gupta V (2023) Erythrocyte Sedimentation Rate. Stat Pearls 4: 1-12.
- Narang V, Grover S, Kang AK, Garg A, Sood N (2020) Comparative analysis of erythrocyte sedimentation rate measured by automated and manual methods in anaemic patients. J Lab Physicians 12(4): 239-243.
- Jeremia IBL (2024) The effect of examination delay on the erythrocyte sedimentation rate in EDTA blood samples. Journal of Evidence Based Nursing and Public Health 1(2):
- Walle M, Addisu T, Mesay A, Getu F, Haftu A, et al. (2024) Comparison of erythrocyte sedimentation rate measurement between Westergren method and automated method among patients attending Jigjiga University Sheik Hassen Yabare Referral Hospital, Jigjiga, Ethiopia. Front Med (Lausanne) 11: 1414097.
- Brigden ML (1999) Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician 60(5): 1443-1450.
- Koepke JA (2002) Welcome innovation in erythrocyte sedimentation testing. Am J Clin Pathol 118(1): 11-12.
© 2025 John Abner Goldman. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






