- Submissions

Full Text
Progress in Petrochemical Science
Green Catalysis Toward Sustainable Processes: Current Progress and Perspectives
Ibtisam Kamal1* and Firas Albadran2
1Petroleum Engineering Department, Basrah University College of Science and Technology, Iraq
2Department of Chemical and Petroleum Refinery Engineering, College of Oil and Gas Engineering, Basrah University for Oil and Gas, Iraq
*Corresponding author:Ibtisam Kamal, Petroleum Engineering Department, Basrah University College of Science and Technology, Iraq
Submission: June 20, 2024;Published: June 28, 2024

ISSN 2637-8035Volume6 Issue3
Abstract
Catalysts are components usually used for the purpose of accelerating the rate of reactions by providing a surface for reactants to adsorb onto and products to desorb from. The role of the catalyst is to lower the activation energy of a reaction by providing a reaction pathway. However, the efficiency of the catalytic process reactions is affected many factors including the porosity and the presence of impurities that can influence the active surface of the catalyst. On another hand, the future of the industries is strictly related to the lessening of its environmental impact and more specifically to the solution of the climate warming problem. Therefore, established systems with sustainable catalysis processes is required for energy preservation and cleaner environment. The review highlights the role of green catalysts in industry and the current progress and perspectives of the industrial applications of some green catalysis processes for achieving the environmental, economic and social sustainability.
Keywords:Catalysis; Sustainability; Green catalyst; Decarbonization; Green processes
Introduction
Hydroxypropyl cellulose is an outstanding non-ionic cellulose ether, prepared by reacting pure cellulose with propylene oxide and a small amount of sodium hydroxide [1]. The introduction of hydroxypropyl group significantly weakens the intramolecular and intermolecular hydrogen bonding force of cellulose, thus enhancing its solubility in solution and endowing it with excellent water solubility [2]. Moreover, through continuous research and development, it has been found that hydroxypropyl cellulose has superior edible, thermoplasticity [3], biocompatibility [4] and degradability [5]. As a result, hydroxypropyl cellulose is extensively used as a food additive [6], binder, thickener [7] and stabilizer [8] in numerous fields such as food, environment, daily chemical, textile and others, making significant contributions to the development of these industries. Hydroxypropyl cellulose, although possessing a significant number of hydroxyl groups that could be utilized as reaction groups, it lacks specific functional groups. This necessitates graft modification, which can produce a novel type of hydroxypropyl cellulose with enhanced functions. This significantly broadens its application field and as such, it presents a considerably broader development prospect.
Sustainable Processes Based on Green Catalysts
Catalysts are crucial in many industrial processes, such as the manufacture of chemicals, petrochemicals, pharmaceuticals and food [1,2]. Biocatalysis plays a pivotal role in the production of chemicals and fuels. Indeed, biocatalysis has numerous benefits to offer in the context of green chemistry and sustainable development. Biocatalyst are green catalysts derived from renewable resources, they are biocompatible (sometimes even edible), biodegradable and essentially non-hazardous, i.e. it fulfils the criteria of sustainability [3]. Biocatalysis avoids the use of scarce precious metals, the long-term commercial viability of which is questionable and at the same time they are ecofriendly.
Enzymes as biocatalysts
Enzymes are substances derived from renewable resources (produced by a living organism). Enzyme acts as a catalyst to bring about a specific biochemical reaction. Enzymes are divided into six functional classes and are classified based on the type of reaction in which they are used to catalyse. The six kinds of enzymes are hydrolases, oxidoreductases, lyases, transferases, ligases and isomerases. Industrial enzymes are enzymes that are commercially used in a variety of industries such as pharmaceuticals, chemical production, biofuels, food and beverage and consumer products [4]. Due to advancements in recent years, biocatalysis through isolated enzymes is considered more economical than the use of whole cells. Enzymes are biocompatible and biodegradable. Enzymatic reactions are performed under mild conditions (physiological pH and ambient temperature and pressure) in high selectivity, affording products in higher purity and processes that are more efficient in energy and raw materials consumption and generate less waste than conventional routes [5]. Figure 1 shows a scheme for bioethanol production through the enzymatic conversion of polysaccharides to glucose.
Figure 1:Production of bioethanol using biocatalysts (enzymes).

Natural soil-based catalysts
Natural soil-based catalysts are another type of green catalysts that have been used extensively. Elements such as Si, Al, Fe, Ca and Ti are abundant in soil and are effective catalysts for biodiesel production [6]. Bentonite, a natural clay, is highly abundant in soil and can potentially function as a catalyst for biodiesel production [7]. Bentonite contains large amounts of montmorillonite, which has a large specific surface area and a net specific charge, which provides extensive ion exchange capacity. The properties of bentonite can be enhanced further by impregnation with an alkali, such as KOH, NaOH, or NaCO3.
Catalytic Processes to Accelerate Decarbonization
Figure 2:Energy diagrams for a single-step reaction in the presence and absence of a catalyst.

Achieving a net zero carbon society is impossible without catalysis; catalysts are central to efficient chemical processes and manufacturing, controlling both the rates and energy demand of chemical reactions. It lowers the activation energy of the reaction, so that more reactant molecules collide with enough energy to surmount the smaller energy barrier and therefore, it shortens the pathway of the reaction without changing the energies of the reactants or products and hence, no change on the free energy (Figure 2). It is well recognized that more than 90% of the chemical processes and thus the majority of all commodities produced involve catalytic transformations. There are many types of catalysts, currently the most studied and most commonly used are those that are derived from transition metals [8]. The dominance of transition-metal catalysts largely results from a combination of their efficiency, distinct modes of reactivity and the predictable control of both activity and selectivity upon ligand modification. These metals are characterized by their general resistance to oxidation and corrosion and consequently are also referred to as noble metals. Precious metals include the elements found in the second and third rows of the periodic table; those commonly used as transition-metal catalysts include rhodium, palladium, platinum, ruthenium, iridium, gold, silver and osmium. For one, the fact that precious metals are by definition scarce indicates that they lack abundance, are very expensive and are susceptible to supply fluctuations, which fuels growing concerns about their continued availability. However, with new catalysis, clean manufacturing and products are developed for the future: fit for a sustainable and hightech set of future industries.
The role of catalysts in reducing CO2 emissions
Reducing carbon dioxide emissions is one of the critical challenges to mitigate global climate change, which is having detrimental impacts on society and the environment. Fossil fuel combustion in transportation, power generation and industrial processes is the dominant contributor to carbon emissions. Numerous strategies have been established to mitigate CO2 accumulation in the atmosphere, among which carbon Capture and Storage/Sequestration (CCS) is considered as a promising CO2 reducing option. In contrast to carbon sequestration, converting CO2 into valuable chemicals could be a sustainable option [9]. Decarbonization have been investigated and developed over the past decades as a sustainable solution to mitigate carbon emissions. Catalysis plays an essential role to address climate change challenge by increasing energy efficiency, reducing carbon emissions, capturing carbon dioxide and utilizing clean energy sources to displace fossil fuels. Decarbonization through the conversion of CO2 to other valuable chemicals has become an interesting topic because it can reduce atmospheric CO2 concentration and produce useful compounds, such as fuels. For example, CO2 could be hydrogenated to methanol, ethanol, dimethyl ether, formic acid, acetic acid, methane, carbon monoxide and gasoline. A typical example is highlighted in the next session.
Hydrogenating of CO2 for methanol production: CO2 is a stable molecule and difficult to convert. However, reacting CO2 with hydrogen can overcome this limitation and generate economically viable fuels including methanol. Methanol is an important chemical feedstock for internal combustion engines and fuel cells, as well as a platform molecule for the production of fine chemicals. Methanol is also a liquid at ambient temperature and pressure, making it easy to store and manage. Methanol was first produced in an industrial setting in 1923. BASF built a methanol manufacturing plant in Germany utilizing syngas (a mixture of CO2 and CO) as feedstock, and ZnO-Cr2O3 system as catalyst at 300-400 °C and 25-30MPa, however, the reaction conditions were too severe. The conventional commercial gas-phase process for methanol production from syngas carries out the conversion in fixed-bed reactors (adiabatic reactor). The three reactions included are highly exothermic. The reactions are presented in equations 1-3.
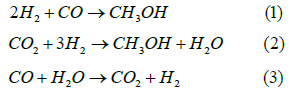
To make the process sustainable, the recycling of H2 rich gas moderates the temperature rise across the adiabatic reactor. CO concentration at the reactor inlet is normally limited to about 10- 15%, after dilution with recycled H2. Figure 3 illustrate a schematic diagram of the process [10]. Other catalyst systems were used for methanol synthesis. A significant amount of methanol is formed recently by reacting syngas with hydrogen over a Cu-ZnO-Al2O3 catalyst at temperatures between 200 and 300 oC, and up to 30bar of pressure. The catalyst can accelerate the chemical reaction by attracting the key gases onto its surface to be reacted and then releasing the products. However, this catalyst is not suitable for converting just CO2 and hydrogen to methanol, due to a limited lifetime and moderate selectivity to methanol. Because of this a variety of alternative catalysts are developed. Recent advances have employed a new catalyst composed of carbon, nitrogen and platinum. This catalyst is a solid material that is suspended in sulfuric acid to aid in the catalysis. The material is easily recyclable as it can be filtered from the acid.
Figure 3:Schematic diagram for methanol production from syngas [10].

One of the sustainable approaches is sourcing green (renewable) instead of grey (non-renewable) CO2 or H2. The green reactants are more costly, but is also expected to gain popularity with time, therefore becoming more affordable. The manufacture of methanol through thermo-catalytic CO2 hydrogenation with “green hydrogen” (derived from renewable energy sources) is regarded as the foundation of the “methanol economy”. This technology is a potential and promising technology for the conversion and utilization of CO2 [11]. Green Liquid CO2 that is obtained from crop-fed biogas plants could be used. On another hand, utilizing a catalyst, especially at the nano-level, significantly enhances surface area, increasing CO2 consumption and fuel generation. It was reported that about 92% syngas conversion per pass and more than 90% selectivity was obtained using Cu nanoparticles as catalyst. The % syngas conversion and % selectivity was found to depend on the amount of the nano Cu catalyst employed at 100 °C and 20bar syngas pressure [12,13].
Conclusion
Catalysts are leaders in the quest for sustainability in catalytical reactions. Their ability to make reactions more efficient, selective and environmentally friendly is transforming industries worldwide. For advancing the field of catalysis, it is essential to look forward sustainable catalysis processes to minimizing the energy consumed and carbon footprint of the reaction products. In the real-world time, green catalysts are crucial components and tools of modern chemistry and chemical engineering for achieving sustainable processes. Nevertheless, challenges persist. The time-consuming and costly processes of the design and development of new catalysts is one of the challenges and the unavailability of catalysts to all the reactions is another challenge. Moreover, the exitance of rare or toxic elements in the catalyst may create resources of environmental pollution. Therefore, more effort is required in research for developing more efficient and novel synthesis routes and advanced characterization methods for green and sustainable catalysts.
References
- Zybert M (2023) Applied catalysis in chemical industry: Synthesis, catalyst design and evaluation. Catalysts 13(3): 607.
- Filippi E, Pizzolitto C (2022) The past and the future of catalysis and technology in industry: A perspective from Casale SA point of view. Catalysis Today 387: 9-11.
- Hughes G, Lewis JC (2018) Introduction: Biocatalysis in Industry. Chem Rev 118(1): 1-3.
- Wu S, Snajdrova R, Moore JC, Baldenius K, Bornscheuer WT (2021) Biocatalysis: Enzymatic synthesis for industrial applications. Angew Chem Int Ed 60(1): 88-119.
- Ismail CA, Dinu CZ (2018) Industrial applications of enzymes: Recent advances, techniques and outlooks. Catalysts 8(6): 238.
- Dai YM, Lin JH, Huang ST, Lee WL, Hsieh CH, et al. (2020) Natural soil and lithium carbonate as economical solid-base catalysts for biodiesel production. Energy Reports 6: 2743-2750.
- Naeem A, Zaman S, Farooq M, Khan IW, Ghazi ZA, et al. (2022) Biodiesel production from waste cooking oil employing natural bentonite supported heterogeneous catalyst: Waste to biodiesel. Korean J Chem Eng 39: 1450-1459.
- Perla SL, Kotolevich Y, Rosario I, Gaxiola JA, Chowdari RK, et al. (2021) Recent advances in catalysis based on transition metals supported on zeolites. Front Chem Sec Catalysis and Photocatalysis 9.
- Fu HC, You F, Li HR, He LN (2019) CO2 capture and in situ catalytic transformation. Front Chem 7: 525.
- Timsina R, Thapa RK, Moldestad BM, Eikeland MS (2021) Methanol synthesis from syngas: A process simulation. Proceedings of SIMS EUROSIM, pp. 444-449.
- Haag S, Welter FC, Schuhmann T, Williams BA, Oelmann T, et al. (2018) How to convert CO2 to green methanol. Challenges for petrochemicals and fuels: Integration of value chains and energy transition DGMK conference, Berlin, Germany.
- Sollai S, Porcu A, Tola V, Ferrara F, Pettinau A (2023) Renewable methanol production from green hydrogen and captured CO2: A techno-economic assessment. Journal of CO2 Utilization 68: 102345.
- Sama CA, Olsbyeb U, Jens KJ (2018) Low temperature methanol synthesis catalyzed by copper nanoparticles. Catalysis Today 299: 112-119.
© 2024 Ibtisam Kamal. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






