- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Post Cardiac Injury Syndrome Following Permanent Pacemaker Implantation-A Rare Entity
Vineet Bhatia1*, Vidit Bhatia2, Kapil Dev Mohindra3 and Parneesh Arora1
1Director, Department of Cardiology, Medanta Hospital, India
2Hamdard Institute of Medical sciences and Research, India
3Associate Director, Department of Cardiology, Medanta Hospital, India
*Corresponding author: Vineet Bhatia, Director, Department of cardiology, Medanta Hospital, India
Submission: September 08, 2025;Published: November 18, 2025

ISSN 2578-0204Volume5 Issue 1
Abstract
Post Cardiac Injury Syndrome (PCIS) encompasses a heterogenous group of immune mediated inflammatory disorders characterized by pericardial and pleural effusion along with raised serum markers of inflammation following recent cardiac injury. They may follow acute Myocardial Infarction (MI), pericardiotomy (open-heart surgery, such as coronary artery bypass surgery or valve surgery) and post-traumatic pericarditis due to accidental or iatrogenic injury such as pacemaker insertion, radiofrequency ablation and coronary angioplasty. Though typically benign they can lead to significant morbidity and even mortality if there is an inordinate delay in diagnosing the problem and initiating appropriate treatment. The following case report highlights an unusual case of PCIS following permanent pacemaker insertion that required active medical management for a successful outcome.
Introduction to TTR Amyloidosis
Cardiac tissue injuries of varied aetiology may trigger a heterogenous group of immune mediated disorders referred to as Post-Cardiac Injury Syndrome (PCIS). When it follows MI, it is referred to as Dressler’s syndrome (first described by William Dressler at Maimonides Medical Center in 1956) [1]. The diagnosis of PCIS (ESC guidelines 2015) [2] requires a history of cardiac injury along with the presence of at least 2 of the following 5 features: fever (in the absence of an alternative cause); pleuritic or pericardial chest pain; pericardial or pleural rub on clinical examination; pericardial effusion; and/or pleural effusion along with elevated inflammatory markers such as C-Reactive Protein (CRP).
PCIS following pacemaker implantation is rare [3]. 1-2% of pacemaker implants may be followed by PCIS [4]. In this case report we delve into the diagnostic protocols and treatment options that exist should this rare complication occur.
Case
An 82 years old male, diabetic and hypertensive presented with syncope to the emergency room. ECG at presentation was suggestive of a ventricular rate of 35/min, Left Bundle Branch Block (LBBB) rhythm with 2:1 AV block. He was on DDP4 inhibitor, metformin combination therapy for diabetes and on amlodipine for hypertension. There was no history of chest pain, breathlessness, or fever at presentation. There was no history of pre syncope or syncope in the past. An acid blood gas analysis in Emergency room revealed normal serum electrolytes. Echocardiogram revealed no regional wall motion abnormality, normal biventricular function, aortic valve sclerosis and no pericardial effusion. He was shifted to the cardiac catheterization laboratory for an urgent Temporary Pacemaker Insertion (TPI) which was carried out successfully under all aseptic precautions from the right femoral venous route. A coronary angiogram revealed non obstructive coronary artery disease. His Kidney Function Tests (KFT) including serum electrolytes, thyroid function test and cardiac enzymes were normal. The next day he continued to be TPI dependent in view of which after an informed consent MRI safe Dual chamber Permanent Pacemaker Implantation (PPI) was done from left subclavian approach (Vitatron, G70A2). Satisfactory lead parameters were achieved at implantation.
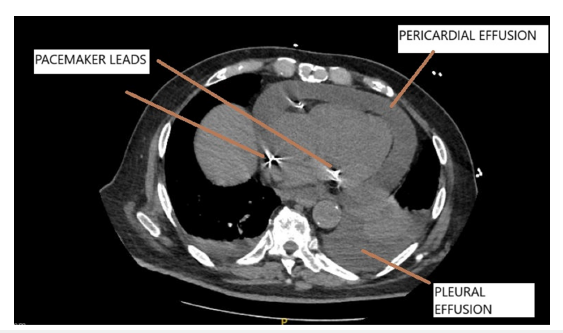
The right ventricular lead had a screw in tip (active fixation) while the lead in right atrial appendage was tined (passive fixation). Post procedure chest X ray was satisfactory and patient was uneventfully discharged on 3rd post op day. Stitch removal was done on 10th post op day and patient had no complaints then. 3 weeks later the patient presented with breathlessness which had rapidly progressed over the last 3-4 days. At presentation he was orthopneic. There was low grade fever, no cough or expectoration and chest x ray revealed blunting of the costophrenic angles. Physical examination revealed a temperature of 100.4-degree Fahrenheit, pulse rate of 130/minute, blood pressure of 110/70mmHg, respiratory rate of 33/minute, and raised JVP with no evidence of pedal edema. Chest auscultation revealed decreased breath sounds at bilateral lung bases and normal heart sounds with evidence hypoxemia (SPO2 of 82% on room air). An echocardiogram revealed moderate circumferential pericardial effusion with maximum localization posterolateral to the left ventricle. Effusion along the right ventricle was mild and pacing leads were seen in the right atrium and right ventricle with mild lead induced tricuspid regurgitation. There was no evidence of cardiac tamponade. On pacemaker interrogation all parameters were found to be satisfactory (lead impedance and threshold were within normal limits). A high-resolution CT scan of the thorax revealed evidence of moderate pericardial effusion, small to moderate pleural effusion and no evidence of extracardiac migration of the pacemaker leads (Figure 1). The fluid in the pericardial cavity had an attenuation value of 40 Hounsfield units suggestive of an exudative nature of the pericardial collection. His complete blood counts, KFT, procalcitonin were normal however ESR was 68mm at the end of 1st hour and CRP was 122.48mg/L. A pleural tap was done under Ultrasound guidance and biochemical examination of the aspirate was suggestive of an exudative etiology using Light’s criteria. The total cells count was 500cu/ mm(P-78.0%) with pleural fluid/serum protein of 0.8 and pleural fluid LDH/serum LDH of 1.1. Pleural fluid ADA level was normal (4IU/L) and TB PCR was negative. Microbiological examination of the Pleural fluid was negative for bacteria, fungus, and tuberculosis and there was no evidence of malignant cells on cytological examination. In view of the above findings the possibility of PCIS though rare was strongly considered. The patient was started on tab Colchicine 0.5mg twice a day. However, by day 10 no significant improvement was noted. In view of the same oral prednisolone 60mg once a day was added following which he showed marked clinical improvement within a week. Prednisolone was tapered over the next 2 weeks and colchicine was discontinued at 2 weeks. All inflammatory parameters normalized and echocardiogram showed complete resolution of pericardial effusion. Patient is doing well at 3 months follow up and review echocardiogram showed no pericardial effusion.
Figure 1:CT chest shows pericardial and bilateral pleural effusion. Pacemaker leads are also seen.
Discussion
PCIS is most, commonly found to occur post cardiac surgery [5]. It usually manifests a few days to weeks post the index procedure with an incidence of 10-40%. With recent advances in cardiac interventions in acute MI settings the incidence post MI PCIS (Dressler’s syndrome) is declining and current estimates suggest an incidence of 5% [6,7]. PCIS occurrence following PPI implantation is rather rare (may complicate 1-2% of implants). This may lead to significant morbidity and rarely mortality due to cardiac tamponade, arrhythmias, pleural effusion, and constrictive pericarditis [6].
Immune mediated mechanisms are proposed to be in play a vital role in the pathogenesis of this heterogenous condition. It is presumed that initial injury to pericardial cells combined with blood in the pericardial space triggers an immune response, resulting in immune complex deposition in the pericardium, pleura, and lungs. The latent period between cardiac injury and clinical onset of symptoms of days to weeks to months, coexistent pleural effusion, and possible pulmonary infiltrates, and increased anti-cardiac antibodies strongly support the above-mentioned hypothesis [8]. However, unlike other autoimmune diseases, circulating anticardiac antibodies are not detected until 14 days after the onset of PCIS, rather than at the initial diagnosis, and thus are not helpful in the diagnosis of PCIS [9].
PCIS following pacemaker implantation is rare and its occurrence is linked to advanced patient age, female sex, use of steroids in the preceding seven days prior to the implant, use of temporary transvenous pacemaker and active fixation atrial leads. Trevor O Green et al reported that 6 out of 123 (4.9%) of their patients developed acute pericarditis following an active fixation atrial lead [10]. The active fixation leads when screwed may cause injury more so in the atrial location as the atria is a thin-walled structure.
The clinical signs and symptoms may be non-specific. The ESC guidelines mentioned earlier may help reach the diagnosis if two of the five criteria are met. There is evidence of pleural and pericardial involvement, markers of inflammation such as CRP and ESR are elevated in 80% cases. Atrial fibrillation, unexplained anaemia and hyponatremia are some of the other presenting features [6].
An echocardiogram helps assess the pericardial effusion, diagnose cardiac tamponade, free wall rupture apart from assessment of the biventricular function. Pacemaker interrogation is vital as an increased capture threshold, a loss of R wave amplitude, and a significant increase or decrease in lead impedance may be indicators of lead perforation. Chest x ray may help diagnose pleural effusion. CT scan of the thorax helps detect pleural and pericardial effusion which usually coexist. Khalid et al reported a unique presentation of PCIS. The patient had with isolated haemorrhagic pleural effusion without pericardial effusion which made the diagnosis even more challenging [11]. The scan may help diagnose lead perforation if the scan is gated. One must be aware of artefacts mimicking lead perforation [12]. The attenuation values of fluid in the pericardial space may help in diagnosing the aetiology of the effusion. A haemorrhage is associated with an attenuation value of >60 Hounsfield units while values <10 and >10 are in favour of transudate and exudative effusions respectively [13]./
Anti-inflammatory medications like Nonsteroidal Anti- Inflammatory Drugs (NSAIDs), aspirin and colchicine form the first line of treatment for patients with PCIS [6]. These drugs are continued until symptoms resolve and then gradually tapered off. The pleural and pericardial effusion are closely monitored and if need be pericardial tapping (in case of tamponade) and thoracocentesis may be undertaken. The fluid aspirated may be subject to suitable biochemical and cytological examination to aid diagnosis. Inflammatory parameters like CRP are also monitored closely. Steroids are used in patients are intolerant or not responding to the first line of treatment which later tapered. Patients of PCIS usually have a favorable prognosis. They may rarely develop constrictive pericarditis at a later stage and hence need to be on close follow up for 6-12 months [6].
Conclusion
PCIS post PPI though rare should be kept in mind if a patient who had undergone the procedure weeks to months ago presents with fever, chest pain and breathlessness. An echocardiogram, CT chest, raised inflammatory parameters, biochemical and cytological evaluation of pleural or pericardial fluid may help establish a diagnosis. Anti-inflammatory agents form the main stay of therapy though at times therapeutic drainage of pleural or pericardial effusion may be necessary (if significant and causing respiratory distress or hemodynamic compromise).
References
- Dressler W (1959) The post-myocardial-infarction syndrome: A report on forty-four cases. AMA Arch Intern Med 103(1): 28-42.
- Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, et al. (2015) 2015 ESC guidelines for the diagnosis and management of pericardial diseases: The task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36(42): 2921-2964.
- Saleem M, Boruah P, Naik S (2021) Post Cardiac Injury Syndrome (PCIS) after placement of permanent pacemaker. JACC 77(18 Suppl 1): 2114.
- Ohlow MA, Lauer B, Brunelli M, Geller JC (2013) Incidence and predictors of pericardial effusion after permanent heart rhythm device implantation: Prospective evaluation of 968 consecutive patients. Circ J 77(4): 975-981.
- Stelzner TJ, King TE, Anthony VB, Sahn SA (1983) The pleuropulmonary manifestations of the postcardiac injury syndrome. Chest 84 (4): 383-387.
- Sarma A (2023) Postcardiac injury syndrome complicated by pleural and pericardial effusion following transvenous pacemaker insertion. IHJ Cardiovascular Case Reports (CVCR) 7(2): 65-67.
- Verma BR, Montane B, Chetrit M, Khayata M, Furqan MM, et al. (2020) Pericarditis and post-cardiac injury syndrome as a sequela of acute myocardial infarction. Curr Cardiol Rep 22(10): 127.
- Patel ZK, Shah MS, Bharucha R, Benz M (2022) Post-cardiac injury syndrome following permanent dual-chamber pacemaker implantation. Cureus 14(1): e21737.
- Zhang R, Du J, Liu M (2023) Post-cardiac injury syndrome occurred two months after permanent dual-chamber pacemaker implantation. BMC Cardiovasc Disord 23(1): 259.
- Greene TO, Portnow AS, Huang SK (1994) Acute pericarditis resulting from an endocardial active fixation screw-in atrial lead. Pacing Clin Electrophysiol 17(1): 21-25.
- Khalid N, Rana IA (2023) Post-cardiac injury syndrome following permanent pacemaker implantation presenting exclusively as massive pleural effusion: A rare occurrence. J Pak Med Assoc 73(10): 2093-2095.
- Morgan H, Willimas C, Blesdale RA (2020) Computed tomography artefact finding of pacing lead perforation. Br J Cardiol 27: 97-99.
- Cetin MS, Ozcan CEH, Ozdemir M, Topaloğlu S, Aras D, et al. (2017) Effectiveness of computed tomography attenuation values in characterization of pericardial effusion. Anatol J Cardiol 17(4): 322-327.
© 2025 Vineet Bhatia. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






