- Submissions

Full Text
Open Access Research in Anatomy
Effects of 6-Gingerol (6-GIN) and Delphidins (DELs) on the Liver of Alloxan (ALX)-Induced Diabetes in Albino Wistar Rats
Joseph MI*
Faculty of Medicine, University of Nigeria, Nigeria
*Corresponding author: Joseph Marcel Ikhenoba, Faculty of Medicine, University of Nigeria, 3a, Edalere Lane, Fadeyi, Yaba, Lagos, Nigeria
Submission: September 22, 2025;Published: December 19, 2025

ISSN: 2577-1922
Volume3 Issue 2
Abstract
The current investigation’s objective is to assess the impact of 6-Gingerol (6-GIN) and Delphidins (DELs) on the liver of an Alloxan (ALX) induced diabetic albino wistar rats. 6-Gingerol (6-GIN) and Delphidins (DELs) juice (60mg/kg body weight) were administered daily to an Alloxan (ALX) induced diabetic rats for 60 days. 6-Gingerol (6-GIN) and Delphidins (DELs) in ALX induced diabetic rats, treatment resulted in a significant (P<0.05). There was reduction in the levels of hepatic markers, lipid peroxidation markers like lipid hydro-peroxides (LOOH) and Thiobarbituric Acid Reactive Substances (TBARS), pro-inflammatory mediators like tumor necrosis factor-alpha (TNF-α), phosphorylated nuclear factor kappa-B-p65 (phospho-NF-B-p65) and cyclooxy. There was considerable (P<0.05) rise in the enzymes hexokinase, glucose-6-phosphate dehydrogenase, catalase, and other enzymatic antioxidants (CAT) and the livers of diabetic rats receiving 6-GIN+DELs treatment were shown to have increase in glutathione (GSH), glutathione peroxidase (GPx), non-enzymatic antioxidants such vitamins A, D, E and K, glutathione (GSH), and anti-apoptotic protein Bcl-x, Bcl-2. Therefore, it may be inferred that the administration of ACNs+6- GIN significantly protected the liver from the antioxidant imbalance, inflammation, and apoptosis caused by hyperglycemia and corrected the imbalance in carbohydrate metabolizing enzymes.
Keywords:Alloxan; Delphidins; Gingerol; Teplizumab; Inflammation; Peroxidation
Introduction
Diabetes is a chronic disease that occurs either when the pancreas does not produce enough insulin or when the body cannot effectively utilize the insulin it produces. Insulin is a hormone that regulates blood glucose. Hyperglycemia, also called high blood glucose or raised blood sugar, is a common effect of uncontrolled diabetes and over time leads to serious damage to many of the body’s systems, especially the nerves and blood vessels. In 2014, 8.5% of adults aged 18 years and older had diabetes. In 2019, diabetes was the direct cause of 1.5 million deaths and 48% of all deaths due to diabetes occurred before the age of 70 years. Diabetes mellitus resulted in 460,000 kidney disease deaths and around 20% of cardiovascular deaths. Between 2000 and 2019, there was a 3% increase in age-standardized mortality rates from diabetes. In lower-middle-income countries, the mortality rate due to diabetes increased 13% [1]. By contrast, the probability of dying from any one of the four main non communicable diseases (cardiovascular diseases, cancer, chronic respiratory diseases or diabetes mellitus) between the ages of 30 and 70 decreased by 22% globally between 2000 and 2019 [2]. The physiological balance between the hepatic synthesis of glucose and the consumption of glucose by peripheral tissues controls blood glucose levels. The primary cause of hyperglycemia in diabetes mellitus is increased hepatic glucose production as a result of insufficient insulin secretion or action. In tissues, hyperglycemia encourages oxidative stress, inflammation, and apoptosis. Additionally, inflammation can increase oxidative stress in tissues. The Zingiberaceae family all contains gingerols and some other phytochemicals. They include the major genera such as Alpinia, Etlingera, Curcuma, Globba, Zingiber, Renealmia, Riedelia, Amomum, Aframomum, Boesenbergia, Hedychium, Hornstedia, and Meisteria. Ginger (Zingiber officinale) is one of the most widely used natural products consumed as a spice and medicine for treating nausea, dysentery, heartburn, flatulence, diarrhea, loss of appetite, infections, cough, and bronchitis.
Experimental studies showed that ginger and its active components including 6-gingerol and 6-shogaol exert anticancer activities against gastro intestinal cancer. Ginger has anti-oxidant, anti-inflammatory, anti-tumor, anti-diabetic, neuro protective, gastro protective, anti-emetic and hepato protective effects. All these herbs have long been used in Chinese and Ayurvedic medicines [3]. On the other hand, delphins (DELs) are watersoluble bioactive compounds of the polyphenol class. DELs can be found in dietary sources which include red and purple berries, grapes, apples, plums, cabbage, or foods containing high levels of natural colorants. Berries significantly exhibit antioxidant, antiinflammatory and anti-cancerous activities [4]. Solvent extraction, the supercritical CO2 extraction method, ultrasonic extraction, and microwave extraction are the methods used to extract delphins (DELs) and 6-gingerol (6-GIN). Column chromatography is widely used in purification of DELs while 6-GIN can be purified using a High- Speed Counter-Current Chromatography (HSCCC). Earlier research by Ashutosh [3] & Luca [5] & shows that DELs and 6-GIN can reduce diabetes in diabetes induced male wistar rats respectively. But these are independent. However, this study’s objective was to assess the combined impact of DELs and 6-GIN on the livers of ALX-induced diabetic albino wistar rats in terms of glucose production, oxidative state, inflammation, and apoptosis. The results have shown a great reduction in diabetes.
Materials and Methods
Chemicals
Monoclonal antibodies for pNF-κB p65, iNOS, COX-2, Bcl-2, and cleaved caspase-3 and secondary antibodies were purchased from Isca Diagnostics Exeter, USA. Biomarkers such as Antibodies for Bax and cytochrome c were purchased from BioLegend Inc. (San Diego, CA) and Abcam, respectively. ALX was procured from Sigma- Aldrich.
Animals
Healthy male albino Wistar rats (170-190g b.w) were obtained from the University of Nigeria, Nsukka Housing Centre. The rats were housed in polycarbonate cages and maintained under constant 12h light and dark cycle, and room temperature at 26±2°C. Rats were fed with standard rat food and water was provided. Before the start of the experiment, animals were acclimatized to laboratory condition for one week. This study was approved by the University of Nigeria, Nsukka Ethics Committee.
Induction of diabetes in male albino wistar rats
Diabetes was induced in rats by administrating a single dose of intraperitoneal injection of ALX (60mg/kg b.w) in a buffer (0.2M citrate buffer, pH 5.0). The ALX induced rats were allowed to drink 7% glucose solution for preventing the drug-induced hypoglycemia. After 4 days of the ALX injection, blood was collected from experimental animals from the tail vein, and the blood glucose level was measured. Diabetics treated rats with fasting blood glucose level of above 220mg/dl were considered diabetic and used for further study.
Induction of diabetes in male albino wistar rats
A total of 30 albino wistar rats were divided into six groups of
five animals each (10 normal rats and 20 diabetic rats). The optimal
dosage of Delphidins (DELs) and 6 gingerol (6 GIN) 60mg/ kg b.w
was fixed.
a) Group 1-Normal Control rats (NC)
b) Group 2-Diabetic Control rats (DC)
c) Group 3-Diabetic+6-gingerol (60mg/kg b.w) treated rats
(D+6-GIN)
d) Group 4-Diabetic+Delphidins (60mg/kg b.w) (D+DELs)
e) Group 5 -Diabetic+6 gingerol+Delphidins (60mg/kg b.w)
(D+6-GIN+ DELs)
f) Group 6-Diabetic+Teplizumab-mzwv (Reference drug)
(7mg/kg b.w) treated rats. (D+TZIELD).
Delphidins (DELs) and 6-gingerol (6-GIN) were administered to ALX-induced diabetic rats orally by intragastric intubation, daily for 60 days.
Biochemical analysis
All of the test rats were decapitated in the early hours of the 61st day in order to make their sacrifices. Experimental animals’ blood was drawn by heart puncture, and serum was separated for hepatic marker enzyme analysis. All experimental rats had their livers removed, and it was cooled with ice-cold saline. The liver was taken and used for histopathology, assessment of lipid peroxidation and antioxidant status, as well as the concentrations of enzymes that help the body break down carbohydrates.
Estimation of liver glycogen
Liver glycogen was evaluated by the method of [6].
Assay of hepatic marker enzymes
The hepatic marker enzymes like Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT) and Alkaline Phosphatase (ALP) in serum were estimated using diagnostic kits (Bio Rad Diagnostics, USA).
Determination of carbohydrate metabolizing enzymes
Activities of hepatic hexokinase, glucose-6-phosphate
dehydrogenase, glucose-6-phosphatase and fructose-1,
6-bisphosphatase were measured by the methods of Caroll [6].
Estimation of lipid peroxidation and antioxidants: Levels
of TBARS and LOOH in the liver were measured by the method
of Ahsan [7]. The activity of SOD was evaluated by the method of
Oztürk & Tarhan [8]. The activity of CAT enzyme was measured by
the method given by Jurczuk [9]. GPx and GSH was evaluated by
the method given by Christine & Joseph [10]. Vitamin C and E were
evaluated by Ali [11].
Histopathology: This was done using Andrej & Michał [12]
technique.
Statistical analysis
All data obtained were expressed as mean±S.D. Statistical significance was evaluated by using one-way ANOVA. P value<0.05 was considered as significant.
Result
Effect of 6-GIN+DELs on liver glycogen
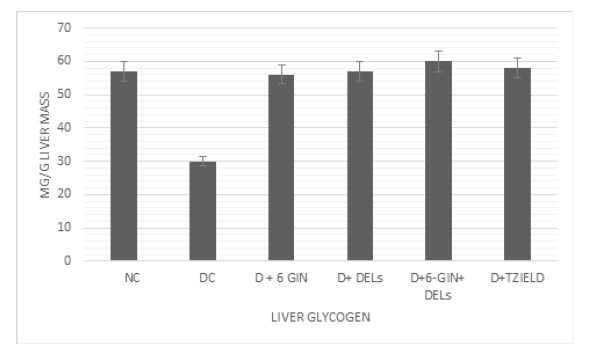
Figure 1 shows the level of liver glycogen in normal and experimental rats. The level of liver glycogen was reduced in diabetic control rats when compared to control rats. Treatment with 6-GIN+DELs had significantly improved glycogen levels in the liver of diabetic rats when compared to diabetic control rats.
Figure 1:Effects of D+6 GIN, D+ DELs, D+6-GIN+ DELs and D+TZIELD on liver glycogen in diabetic rats. All the data are expressed as the mean±S.D. for 6 rats. The results are significantly different at p < 0.05.
Effect of 6-GIN+DELs on hepatic markers
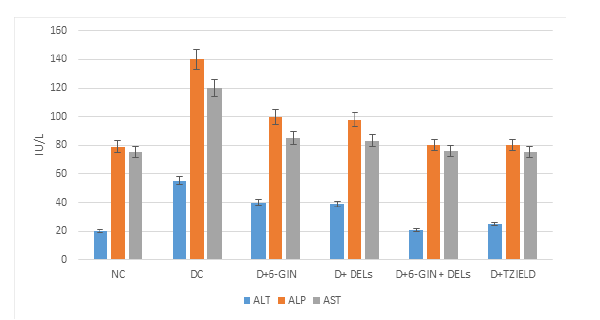
Figure 2 shows the level of hepatic markers such as AST, ALT and ALP in the serum of control and experimental rats. In diabetic control rats, the level of ALT and ALP were significantly (p<0.05) elevated when compared to normal rats. 6-GIN+DELs (60mg/kg b.w) treated groups had a significantly lower level of the hepatic markers than in diabetic control rats.
Figure 2:Effects of NC, DC, D+6 GIN, D+ DELs, D+6-GIN+ DELs and D+TZIELD on the hepatic markers in diabetic rats. All the data are expressed as the mean±S.D. for 6 rats. The results in each experimental groups are significantly different at p < 0.05.
Effect of 6-GIN and ALX on carbohydrate metabolizing enzymes
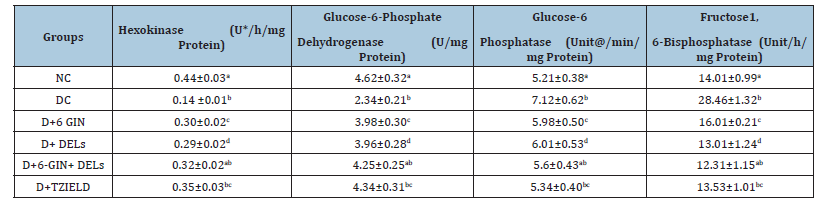
Table 1 depicts the level of carbohydrate metabolizing enzymes in control and experimental rats. The activity of hepatic hexokinase and glucose-6-phosphate dehydrogenase were significantly declined, whereas glucose-6-phosphatase and fructose-1, 6-bisphosphatase activity were significantly elevated in diabetic control rats. The abnormal levels of carbohydrate metabolizing enzymes were found to be significantly improved by 6-GIN+DELs (60mg/kg b.w) or Teplizumab-mzwv (TZIELD) treatment than in diabetic control rats.
Table 1:Effect of 6-Gingerol and Delphidins on the liver carbohydrate metabolizing enzymes in diabetic rats. All the data are expressed as the mean±S.D. for 6 rats. p<0.05.
Effect of 6-GIN+DELs on lipid peroxidation
Table 2 represents TBARS and LOOH levels in the liver of control and experimental rats. TBARS and LOOH levels were significantly (p<0.05) elevated in diabetic control rats when compared to normal control rats, whereas diabetic rats treated with 6-GIN+DELs or TZIELD significantly inhibited the elevated level of TBARS and LOOH as compared with diabetic control rats.
Table 2:Effect of berberine chloride on lipid peroxidation markers, enzymatic and non-enzymatic antioxidants in the liver of diabetic rats. All the data are expressed as the mean±S.D. for 6 rats. p<0.05.
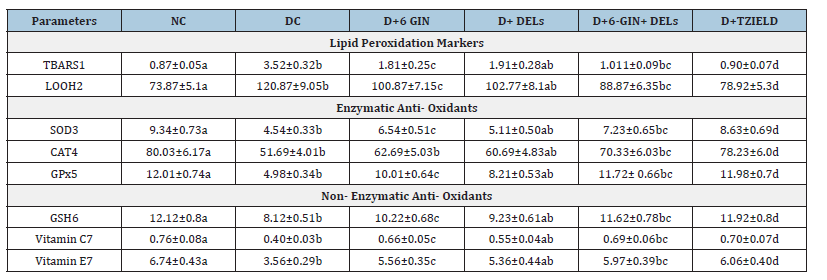
Effect of 6-GIN+DELs on enzymatic antioxidant enzymes: When compared to normal control rats, the activity of antioxidant enzymes like SOD, CAT, and GPx was dramatically reduced in diabetes-induced animals. When compared to diabetic control rats, diabetic rats treated with 6-GIN+DELs or TZIELD show a considerable improvement in the activity of SOD, CAT, and GPx in the liver tissue (Table 2).
Effect of 6-GIN+ALX on non-enzymatic antioxidants
The quantity of non-enzymatic antioxidants in the liver tissue of experimental and control rats is displayed in Table 2. When compared to normal rats, the levels of GSH, vitamin C, and vitamin E were decreased in diabetic control rats. In diabetic rats treated with 6-GIN+DELs or TZIED, these aberrant levels of non-enzymatic antioxidants rapidly improved to levels close to normal.
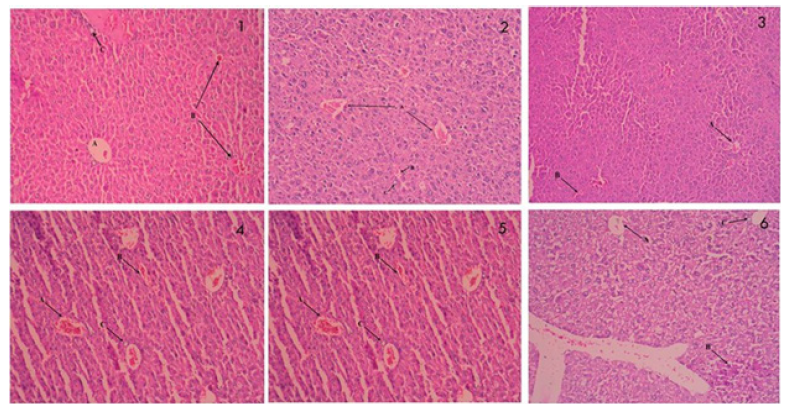
Effect of 6-GIN+DELs on the liver’s histology: The photomicrographs of the liver tissue slices from the control and experimental rats stained with Hematoxylin and eosin are shown in Figure 3. The cellular organization of diabetic control rats was abnormal, there was vacuolization, there were hypertrophy cells, there was inflammation, there was enlargement of the intercellular sinusoids, and there was obstruction of the central vein. Compared to diabetic rats, diabetic rats treated with 6-GIN+DELs (60mg/kg b.w.) or TZIELD.
Figure 3:Effect of D+6 GIN, D+ DELs, D+6-GIN+ DELs and D+TZIELD on histology of the liver in diabetic rats: Photomicrographs of histological changes in Hematoxylin and eosin (H & E) stained liver sections of control and experimental rats (x100). a) 1&3-Normal Control and Alloxan treated rats’ liver showing clear central vein (CV). All hepatocytes are arranged as trabeculae from the central portal vein, which are separated by sinusoids (S), b) 2-Alloxan (ALX) treated rat liver showing deformed cellular organization accompanied with vacuoles (V), hypertrophic cells, Cellular Damage (CD), inflammation, widening of intercellular sinusoids (S) and congestion in the Central Vein (CV). c) 4-6-GIN treated ALX rat liver showing near normal hepatocyte arrangement and sinusoids (S) accompanied with mild Central Vein Congestion (CV). d) 5-D+DELs treated ALX rat liver showing near normal hepatocyte arrangement and sinusoids (S) accompanied with mild Central Vein Congestion (CV). e) 6-D+6-GIN+ DELs treated ALX rat liver showing near normal hepatocyte arrangement and sinusoids (S) accompanied with mild Central Vein Congestion (CV).
Effect of 6-GIN+DELs on TNF-α, NF-κB p65, phospho-NF-κB p65, COX-2 and iNOS protein expressions: TNF-α, NF-B p65, phospho NF-B p65, COX-2, and iNOS protein expression in the liver of control and experimental rats is shown in Figure 4. When compared to normal control rats, the diabetic control rats showed higher levels of the proteins TNF-, NF-B p65, phospho-NF-B p65, COX-2, and iNOS. When 6-GIN+DELs (60mg/kg b.w.) or TZIELD were given to diabetic rats, the levels of TNF-, NF-B p65, phospho-NF-B p65, COX- 2, and iNOS proteins significantly decreased.
Figure 4:Western blot analysis of TNF-α, pNF-κB p65, COX-2 and iNOS in the liver of diabetic rats. Representative Immunoblot analysis. Protein samples (60μg/lane) resolved on SDS-PAGE was probed with corresponding antibodies. Β-Actin was used as loading control. a) Lane 1-NC, b) Lane 2-D, c) Lane 3-D+6-GIN, d) Lane 4-D+ DELs, e) Lane 5-D+6-GIN+DELs, f) Lane 6-D+TZIELD
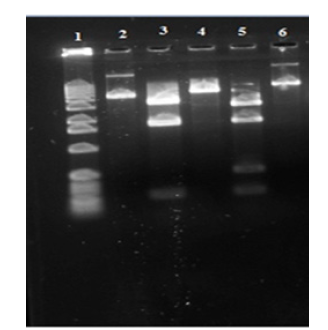
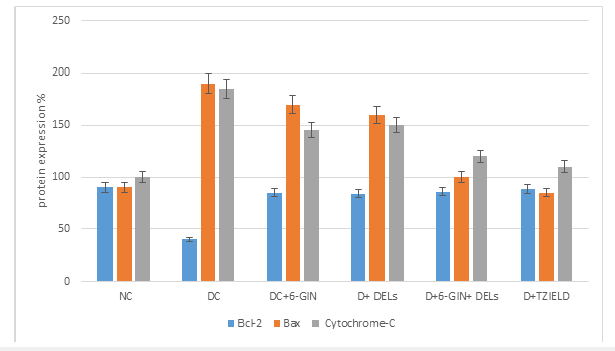
Effect of 6-GIN+ALX on Bax, Bcl-2 and cytochrome c protein expression
Figure 5 displays the RIBA results for Bax, Bcl-2, and cytochrome c in the livers of control and experimental rats. In contrast to diabetic control rats, Bax and cytochrome c protein expression was noticeably increased whereas Bcl-2 expression decreased. When compared to diabetic control rats 6-GIN+DELs -treated diabetic rats had considerably lower levels of the proteins Bax and cytochrome c, while Bcl-2 protein expression had increased. It was discovered that the levels of protein expression in diabetic rats treated with 6-GIN+DELs and TZIELD were comparable.
Figure 5a:Densitometric analysis. The mean protein expression from control lysates for five determinations was designated as 100% in the graph. Mean±SD of six determinants is represented in graph for each group. Significantly different from untreated control (p<0.05).
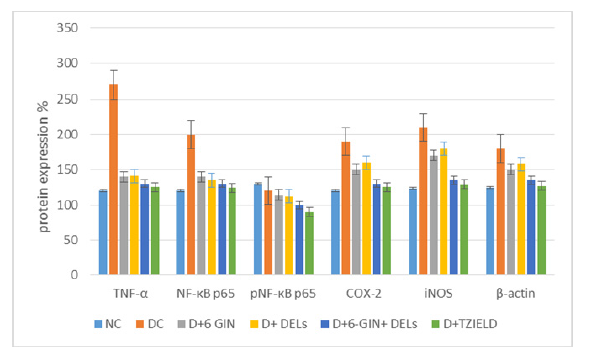
Figure 5b:Western blot analysis of Bax, Bcl-2 and cytochrome c protein expression in the liver of diabetic rats. Representative Immunoblot analysis. Protein samples (60μg/lane) resolved on SDS-PAGE was probed with corresponding antibodies. β-Actin was used as loading control. a) Lane 1-NC, b) Lane 2-D, c) Lane 3-D+6-GIN, d) Lane 4-D+ DELs, e) Lane 5-D+6-GIN+DELs, f) Lane 6-D+TZIELD.
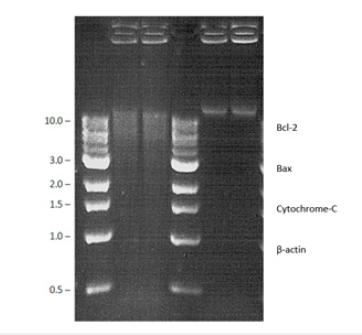
Figure 5c:Densitometric analysis. The mean protein expression from control lysates for five determinations was designated as 100% in the graph. Mean±SD of six determinants is represented in the graph for each group. Significantly different from untreated control (p<0.05).
Effect of 6-GIN+ALX on iNOS and cleaved caspase-3 immunohistochemistry staining in the liver
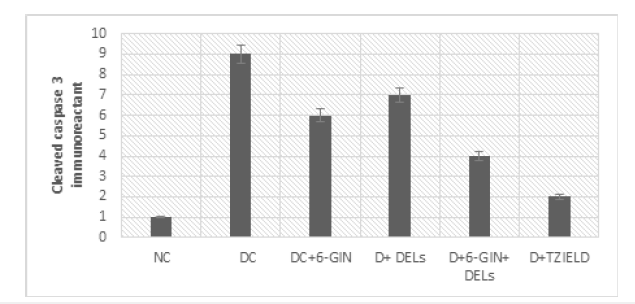
The immune histochemical photomicrographs of iNOS and cleaved caspase-3 in the liver of control and experimental rats are shown in Figure 6 & 7. iNOS and cleaved caspase-3 immunoreactivity were discovered to be increased in the livers of diabetic control rats compared to normal control rats. In diabetic rat liver treated with 6-GIN+DELs and TZIELD, less iNOS and cleaved caspase-3 immunoreactivity was seen than in diabetic control rats.
Figure 6:Effect of 6-GIN and DELs on immunohistochemical staining of iNOS in the liver of diabetic rats (100 X): a) Normal control rats, b) Diabetic Control rats (DC) c) D+6-GIN (D) D+ DELs d) D+6-GIN+ DELs and e) D+TZIELD
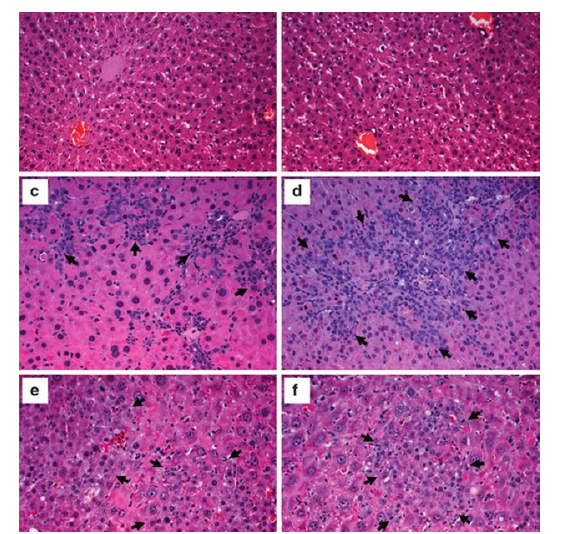
Figure 7a:Intensity of immunoreaction of iNOS in the liver of control and experimental rats: Intensity of the immunoreaction in each group was measured by the image analyzing system (DOB, Ottawa, Canada) and recorded with the range from 0 to 10, mean±SD. Each value is mean±SD for six rats in each group. Significance was accepted at p<0.05 (Tukey multiple comparison test).
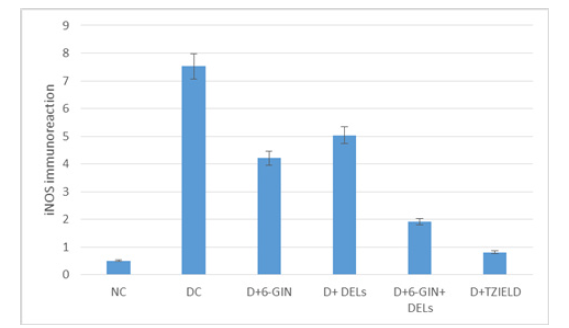
Figure 7b:Intensity of immunoreaction of cleaved caspase-3 in the liver of control and experimental rats: Intensity of the immunoreaction in each group was measured by the image analyzing system (LTB, MA, USA) and recorded with the range from 0 to 10, mean±SD. Each value is mean±SD for six rats in each group. Significance was accepted at p<0.05 (Tukey multiple comparison test).
Discussion
In the diabetic condition, excessive levels of glucose (hyperglycemia) are produced as a result of insulin shortage or inactivity, which can increase oxidative stress and lead to inflammation, apoptosis, and other symptoms. Therefore, in our work, we looked at the effectiveness of 6-GIN and DELs treatment over problems brought on by hyperglycemia on the liver of ALXinduced diabetic rats [13]. Alloxan (60mg/kg b.w) was utilized in this investigation to induce diabetes because it specifically destroys pancreatic beta cells while leaving other cells unharmed. The treatment with a naturally available compound that has free radical scavenging and antioxidant activities may protect the pancreatic islets against the ALX induced diabetic effects. The treatment with a naturally available compound that has free radical scavenging and antioxidant activities may protect the pancreatic islets against the STZ induced diabetic effects. Treatment with 6-GIN and DELs significantly increased the liver glycogen levels in diabetic animals, which may be the result of increased insulin levels. Hepatic markers like AST, ALT, and ALP were found to be significantly higher in ALX-induced diabetic controls, which may be related to enhanced inflammation and liver cell necrosis that was facilitated by the elevated blood glucose levels. As a result, an increase in these markers is primarily a sign of liver damage since they show that ALX is having a hepatotoxic effect on the liver by leaking out of the cytoplasm of hepatocytes and into the bloodstream. These liver indicators decreased levels in the diabetic rat treated with 6-GIN and DELs demonstrated their hepato-protective properties. TBARS and LOOH are common lipid peroxidative markers that are found elevated in ALX induced diabetic rat model. In this investigation, diabetic control rats showed increased levels of TBARS and LOOH. These results indicate elevated levels of oxidative stress in the liver of diabetic control rats.
Treatment with 6-GIN and DELs in ALX-induced diabetic rats significantly reduced the levels of TBARS and LOOH in the liver, indicating antioxidant properties of 6-GIN and DELs. Anti-oxidant enzymes like SOD, CAT, and GPx serve as barriers to the free radical process. 6-GIN and DELs increases these anti-oxidants in ALX-induced rats. In addition, when the cell has increased levels of SOD without a proportional increase in peroxidases (GPx), cells face a peroxide overload. Vit. C, Vit. E and GSH are also elevated by 6-GIN and DELs which reduces oxidative stress and endogenous scavenging. The gluconeogenic pathway’s regulating enzymes include fructose-1, 6-bisphosphatase and glucose-6- phosphatase. The increased glucose synthesis during diabetes may be attributed to the activity of these two enzymes. It has been noted that diabetic rats have considerably higher hepatic glucose- 6-phosphatase and fructose-1, 6-bisphosphatase activity levels. In ALX-induced diabetic rats, oral administration of 6-GIN and DELs dramatically reduced the activities of glucose-6-phosphatase and fructose-1, 6-bisphosphatase, which may have assisted in reducing the generation of glucose from non-carbohydrate sources. In this investigation, the activity of TNF-α, phospho NF-κB p65, COX-2 and iNOS protein expression levels were elevated.
In ALX-induced diabetic rats when compared to the normal control rats. The increased levels of TNF-α, phospho-NF-κB p65, COX-2 and iNOS proteins were significantly reduced in the 6-GIN and DELs or TZIELD treated diabetic rats. These effects may be a result of BC’s ability to prohibit TNF-α, COX-2 and iNOS expressions, by suppressing the activation of NF-κB p65. NFtarget B’s gene, the Bcl-2 protein family, is an essential regulator of the common pathway of apoptosis. According to the findings of this study, an imbalance between NF-B and oxidative stress is to blame for the higher amounts of Bax and cytochrome c found in the livers of diabetic control rats. From my findings, it shows that 6-GIN and DELs therapy significantly lowered the levels of Bax and cytochrome c as well as NF-B activation, which was demonstrated by the decreased level of phosphorylated NF-B p65 in diabetic treated rats. Additionally, it was discovered that diabetic rats treated with 6-GIN and DELs had higher levels of the anti-apoptotic Bcl-2 protein expressed in their livers than diabetic control rats. Animal studies indicate that ginger ethanol extract helps reduce triglycerides and cholesterol in the liver. What is more, ginger may relieve inflammation and reduce levels of inflammatory markers in the liver, such as IL-6 or TNF alpha [14].
Conclusion
Also demonstrating the anti-apoptotic effect of 6-GIN and DELs is the immunohistochemical labeling of cleaved caspase-3 in the liver of diabetic rats treated with 6-GIN and DELs, which was discovered to be a very tiny region. ZBN restored ISO-mediated antioxidant status, increased level of high-density lipoprotein cholesterol (HDL-C), and tissue phospholipids to the near-normal levels. Besides, ZBN pre-treatment significantly reduced the level of inflammatory markers (TNF-α, IL-6, NF-κB, and IL-1β) in ISOinduced MI in rats. We noticed that ZBN pre-treatment inhibited the pro-apoptotic proteins Bax and cytochrome c and increased the Bcl-2 expression in ISO induced rats [15]. All of the aforementioned benefits can therefore be attributed to 6-GIN and DELs’ antioxidant, anti-apoptotic, and anti-inflammatory capabilities.
References
- GBDS (2019) GBD results, Institute for health metrics and evaluation.
- WHO (2022) World diabetes day.
- Ashutosh KY, Reetu, Arun Garg (2019) Antidiabetic effects of [10]-gingerol in streptozotocin-and high-fat diet-induced diabetic rats. Asian Journal of Pharmaceutical and Clinical Research 12(11).
- Akhunzada B, Muhammad I, Munkh AG, Abdul Q, Rujie SS, et al. (2021) A review of the bioactive ingredients of berries and their applications in curing diseases. Food Bioscience 44: 101407.
- Luca V, Patrizia R, Alessandra M, Marisa P, Silvia F, et al. (2013) Dietary anthocyanins as nutritional therapy for nonalcoholic fatty liver disease. Oxid Med Cell Longev 20(2): 145421.
- Carroll NV, Longley RW, Roe RH (1956) The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem 220(2): 583-593.
- Ahsan G, Celia B, Danny RB, Paul DP (2017) Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem 230: 195-207.
- Ozturk UR, Tarhan L (2001) Purification and characterization of superoxide dismutase from chicken liver. Biochem Mol Biol 128(2): 205-212.
- Jurczuk M, Brzóska JM, Moniuszko JM, Gałazyn SE, Kulikowska K (2004) Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol 42(3): 429-438.
- Christine JW, Joseph JC (2009) Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5(1): 51-66.
- Ali ZK, Mustafa Z, Oktay A, Yasar N, Huseyin V (2004) Effects of vitamin C and E on Liver enzymes and biochemical parameters of rabbits exposed to Aflatoxin B1. Vet Hum Toxicol 46(4): 190-192.
- Andrzej G, Michał K (2011) Proposed protocol for histopathological examination of liver specimen in diagnosing chronic hepatitis.
- Sahdeo P, Amit KT (2015) Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterology Res Pract 2015: 14297.
- Xiao HL, Kristine CM, Srinivas N, Alison KH, Basil DR (2011) Attenuation of liver pro-inflammatory responses by zingiber officinale via inhibition of nf-kappa b activation in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol 110(3): 238-244.
- Jianwei L, Radhiga T, Kanimozhi G, Jianxia W (2021) Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals. Hum Exp Toxicol 40(6): 915-927.
© 2025 Joseph MI. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






