- Submissions

Full Text
Modern Research in Dentistry
Biocompatibility Evaluation of Two Endodontic Experimental Medications Containing Calcium Hydroxide and Aloe Vera
Larissa Bertassi1, Carolina Santinoni2* and Graziela Mori1
1University of Western São Paulo, Unoeste, Brazil
2Department of Dentistry, Federal University of Santa Catarina-UFSC, Brazil
*Corresponding author: Carolina dos Santos Santinoni, Department of Dentistry, Federal University of Santa Catarina-UFSC, R Delfino Conti, 1240-Florianópolis, SC, Brazil
Submission: September 11, 2025;Published: November 19, 2025

ISSN:2637-7764Volume8 Issue5
Abstract
Objective: This study evaluated the biocompatibility of two experimental intracanal medications
containing calcium hydroxide and aloe vera in the subcutaneous tissue of rats.
Materials and methods: This study was conducted on fifteen male rats. Two incisions were performed
on the dorsal region of each animal for the introduction of four sterile polyethylene tubes. One tube
was empty (NC), one was filled with zinc oxide-eugenol cement (PC), one was filled with experimental
medication 1 (CH/Aloe gel) and the last tube was filled with experimental medication 2 (CH/Aloe power).
In sequence, the incisions were closed with Nylon 5-0 sutures. After 7, 14 and 30 days, the animals were
euthanized and the specimens were submitted to histo-technical preparation. The histological sections
were analyzed by light microscopy and scores were established according to the inflammatory process.
The data were statistically compared using Kruskal-Wallis’ test and Dunn’s post-test test (p<0.05).
Result: The analysis of the histological sections indicated moderate to severe inflammatory reactions in
the connective tissue in contact with CH/Aloe gel at 7 and 14 days and mild to moderate reactions at 30
days (p>0.05). The tissue in contact with CH/Aloe power exhibited moderate to a severe inflammatory
reaction in all study periods. The statistical analysis indicated a significant difference between both
experimental medications and negative control (p<0.05).
Conclusion: The experimental pastes were not biocompatible with the tissue and new formulations with
changes of components and their concentrations must be performed to justify the use of Aloe vera in
calcium hydroxide pastes.
Keywords:Aloe; Biocompatible materials; Calcium hydroxide
Introduction
Calcium Hydroxide (CH) is indicated to Endodontics in diverse clinical situations, being widely used like intracanal medication [1-3]. One characteristic of this product is its capacity to dissociate into calcium and hydroxyl ions [1,4,5] which permit CH has many effects, including the inhibition of root resorption [1,2,4], antimicrobial action [1,3,5-8] and the ability to induce the formation of mineralized tissue [1,3,9]. Ordinarily, CH is associated with vehicles for the manufacture of pastes which facilitates its clinical use [1,10,11]. Vehicles such as saline, distilled water, glycerin, propylene glycol, chlorhexidine and propolis are employed in CH pastes [1,5,7,10-12]. These vehicles may affect the dissociation of calcium ions and hydroxyl ions [1,10-12] or/and enhance the antimicrobial [1,12] and mineralized [1] capacity of pastes. Aloe vera has been used clinically because of its ability to induce tissue healing [13-17], antiinflammatory activity [16,18,19], the anti-resorptive effect [16,18] and antimicrobial actions [13,14,16,18,19]. One important component of Aloe vera is a polysaccharide called Acemannan [9,13-15]. This promotes the activation of macrophages and productions of cytokines that increase phagocytic activity [13,19], stimulates the secretion of vascular [13,19] and bone [15,19] growth factors, proliferation of fibroblasts [15,19], deposition of collagen [13,19] and minerals [15,19]. These characteristics are significant to an intracanal medication, suggesting using Aloe vera as a component of CH pastes. Batista et al. [20] idealized two experimental CH pastes containing Aloe vera and analyzed the capacity of these in diffusion through the dentinal tubules. The authors concluded that experimental pastes were able the diffuse in dentin and reach the external surface of the root, being an encouraging association to use in endodontics. Consequently, the biological assessment to determine the biocompatibility of those pastes with tissue is essential since they can be contacted with the apical and periapical tissues and interfere in the repair of this region [21]. Hence, the present study evaluated the biocompatibility of two experimental pastes containing CH and Aloe vera in the subcutaneous tissue of rats.
Materials and Methods
Ethical approval
The present study approved by the Institutional Review Board on Animal Experimentation (Protocol 1381) was conducted on 15 male rats (Rattus norvegicus albinus, Wistar) weighing 180- 200 grams. These animals were kept in cages which were cleaned daily and identified with experimental groups and periods. The animals fed solid food and water ad libitum, except for 12 hours preoperatively, when the animals were given water only.
Experimental groups
In the present study, 60 sterile polyethylene tubes 1.3mm in internal diameter and 10mm in length were selected and divided into four groups: Group NC (Negative Control): n=15: empty polyethylene tubes; Group PC (Positive Control): n=15: polyethylene tubes filled with zinc oxide and eugenol (Biodinâmica, Lobato, PR, Brazil). This material was manufactured according to fabricant recommendations; Group CH/Aloe gel (Experimental medication 1): n=15: polyethylene tubes filled with CH p.a (Biodinâmica, Lobato, PR, Brazil) and Aloe vera gel (Botica Magistral, Presidente Prudente, SP, Brazil); Group CH/Aloe power (Experimental medication 2): n=15: polyethylene tubes filled with CH p.a (Biodinâmica, Lobato, PR, Brazil), Aloe vera powder (Botica Nativa, Presidente Prudente, SP, Brazil) and propylene glycol (Botica Magistral, Presidente Prudente, SP, Brasil). Both experimental medications were manipulated according to Batista et al. [20].
Surgical procedures
For surgical interventions, the animals were anesthetized with a combination of ketamine hydrochloride (Sespo Indústria e Comércio Ltda, São Paulo, Brazil) and xylazine hydrochloride (Agri brands do Brasil Ltda, São Paulo, Brazil) by intramuscular injection at a dose of 0.05ml/100g of weight for each substance. Anesthesia was delivered using a disposable insulin syringe. Subsequently, the rats were submitted to trichotomy and the antisepsis of the dorsal region was executed with gauze moistened with 0.12% chlorhexidine (Periogard, Pfizer Ltda, São Paulo, Brazil) followed by the saline solution to remove any chlorhexidine residue. After two incisions were made on the median dorsal region (upper and lower dorsal region) of each rat using a no. 15 blades (Embramac Exportação e Importação, São Paulo, Brazil) and laterally of those, the cutaneous tissue was pinched and was dissected using blunt-ended scissors. Then, the tubes organized according to the experimental groups were introduced into the subcutaneous tissue, immediately after being filled with the test materials Each animal received four tubes being two in the upper dorsal region (NC to the left of the incision and CH/Aloe power to the right) and two in the lower dorsal region (PC to the left of the incision and CH/ Aloe gel to the right). Then, the incisions were closed with Nylon 5-0 sutures (Ethicon, Johnson and Johnson, São José dos Campos, São Paulo, Brazil).
Histological procedures and data analysis
After 7, 14, and 30 days, five animals were euthanized by administering an anesthetic overdose. The subcutaneous tissues were removed, and the samples were fixed in 10% neutral formalin for 48 hours. In sequence, the tubes were removed from the tissue and the specimens were embedded in paraffin. Semi-serial longitudinal sections with 5 micrometers in thickness were performed and stained with hematoxylin and eosin and were analyzed by light microscopy. The histological sections were analyzed regarding the presence and type of inflammatory process, the proliferation of connective tissue, or the occurrence of destructive processes, such as abscess or tissue necrosis. The intensity of inflammation was classified by established scores, which varied according to the intensity of the inflammatory process: score 1=non-significant; score 2=mild; score 3=moderate; and score 4=severe. These criteria were described previously by Mori et al. [22]. The scores were assigned by an experienced examiner blinded to the experimental groups and they were recorded on specific spreadsheets. The data were statistically analyzed using the Jamovi software [23] by the Kruskal-Wallis’ test and Dwass-Steel-Critchlow-Fligner pairwise comparisons at a significant level of 5% (p<0.05).
Result
NC group-negative control
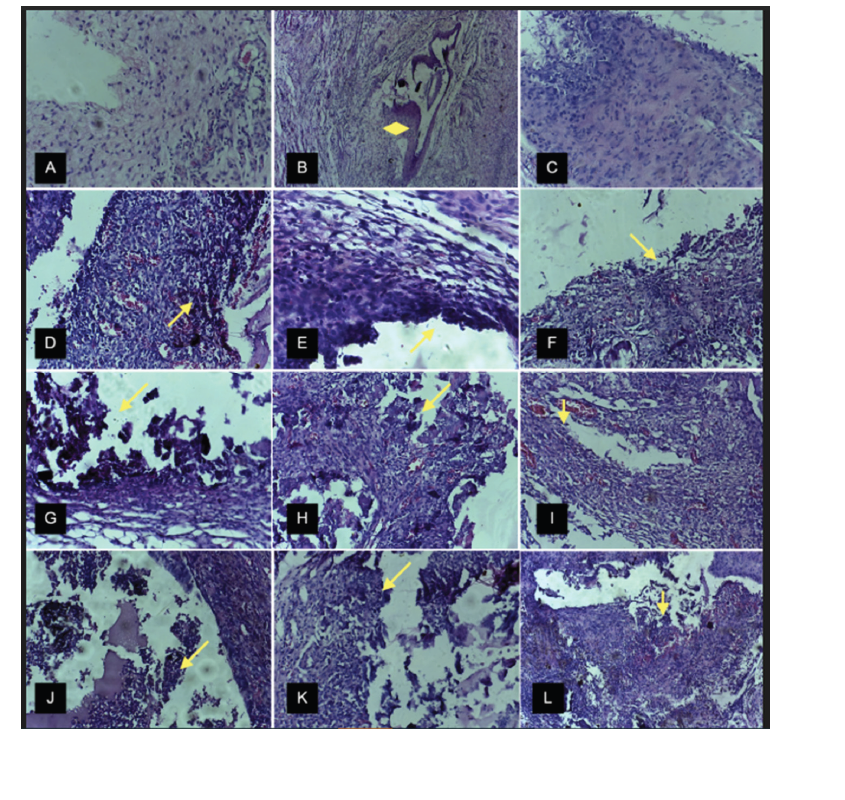
The analysis of the histological control sections confirmed the biocompatibility of the tubes with the connective tissue. At 7 days, there were poorly organized collagen fibers and young fibroblasts. Small areas exhibited neutrophils and clotting regions (Figure 1a). On 14 and 30 days, the connective tissue was well organized, with collagen fibers, fibroblasts, and few blood vessels, with an absence of inflammatory cells (Figure 1b & 1c). In several sections, there was a large amount of proliferation of dense connective tissue toward the inner part of the tube. In all the study periods, the inflammatory process was non-significant or mild without statistically significant differences (p>0.05), confirming the biocompatibility of tubes used in the present study.
PC group-positive control
Microscopic analysis of the histological sections confirmed the irritability of zinc oxide eugenol to the connective tissue. At 7 days, there were noticeable blood clots and inflammatory cells, especially neutrophils. Some sections exhibited poorly organized collagen fibers and young fibroblasts and other tissue sections presented abscess formation (Figure 1d). At 14 and 30 days, the connective tissue displayed few collagen fibers, fibroblasts, or blood vessels. Large quantities of neutrophils and macrophages were observed (Figure 1e & 1f). The scores in all experimental periods indicated a moderate to severe inflammatory process without statistically significant differences (p>0.05). These data confirmed the irritability of this material used as a positive control in this study.
CH/aloe gel group-experimental medication 1
On 7 and 14 days, the sections had few collagen fibers and fibroblasts and blood vessels but large quantities of inflammatory cells (Figure 1g & 1h). At 30 days, several wounds exhibited collagen fibers, fibroblasts, areas of bleeding with vascular growth, macrophages and fibroblasts (Figure 1i). The analysis of the histological sections in this group showed a moderate to severe inflammatory process at 7 and 14 days and a mild to moderate process at 30 days. The was not statistically significant difference among experimental periods (p>0.05).
CH/aloe power group-experimental medication 2
At 7 days, histological sections showed bleeding in some areas, large numbers of neutrophils and some macrophages, a proliferation of tissue and blood vessels (Figure 1j). At 14 days, the sections were characterized by many collagen fibers and fibroblasts, a moderate number of macrophages and the presence of encapsulation material (Figure 1k). At 30 days, the sections were characterized by many inflammatory cells, areas of hemorrhaging, congested vessels, and the presence of phagocytosed materials (Figure 1l). The scores of the groups in all experimental periods indicated a moderate to severe inflammatory process without statistically significant differences (p>0.05).
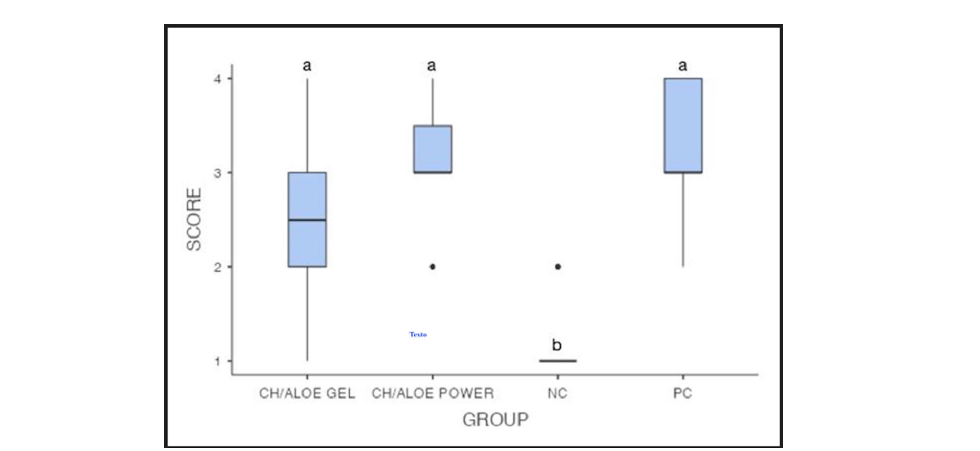
Figure 1:Box plot to scores of the inflammatory process to each experimental group. CH/Aloe gel group, CH/ Aloe gel power group, NC group and PC group represent experimental medication 1, experimental medication 2, negative control and positive control, respectively. The scores describe the intensity of the inflammatory process: score 1=non-significant; score 2=mild; score 3=moderate; and score 4=severe. Different letters express statistically significant differences among groups (Kruskal-Wallis at p<0.001 and Dwass-Steel-Critchlow-Fligner pairwise comparisons).
Comparisons among the experimental groups
The score attributed to each experimental group according to the experimental period can be verified in Figure 2. The CH/Aloe gel and CH/Aloe power groups were statistically different from the NC group (p<0.05) and no different from PC group (p>0.05). These demonstrated the irritability of both experimental medications.
Figure 2:Description of histological sections: A (H&E, x40), B (H&E, x20) and C (H&E, x40)-connective tissue with the absence of an inflammatory process in NC group (negative control) at 7, 14 and 30 days, respectively; In B can be noticed the proliferation of connective tissue toward the inner part of the tube (diamond). D (H&E, x40), E (H&E, x40) and F (H&E, x40)-connective tissue with an inflammatory process (arrow) in PC group (positive control) at 7, 14 and 30 days, respectively. G (H&E, x40), H (H&E, x40) and I (H&E, x40)-connective tissue with the presence of an inflammatory process (arrow) in CH/Aloe gel (experimental medication 1) group at 7, 14 and 30 days, respectively; In I can be observed the mild inflammatory process. J (H&E, x40), K (H&E, x40) and L (H&E, x20)-connective tissue with an inflammatory process (arrow) in CH/Aloe power (experimental medication 2) at 7, 14 and 30 days, respectively.
Discussion
To be accepted and applied in endodontic clinical, intracanal medication should present characteristics such as antimicrobial activity, biocompatibility and the promotion of repair [21,22,24-29]. Several tests may be used to evaluate the reaction of cells and tissues to the presence of materials, including measures of cytotoxicity and genotoxicity and the use of subcutaneous implants or bone implants [24-29]. The subcutaneous implantation of materials in rats had been used frequently and their results may be extrapolated to clinical practice [24-28]. The implantation method is a valid test to investigate the biocompatibility of materials. In this method, the material is introduced into the animal’s tissue using polyethylene tubes; the latter are not irritating and may prevent the spread of the former, simulating the clinical conditions of medications are used in the root canal space [22,24,26-28]. A biocompatible material should present low toxicity without promoting an inflammatory reaction and when one is present, it should be non-significant or mild when present. The material can be considered biocompatible if the inflammatory process decreases to non-significant levels in a reasonable amount of time [22,24]. According to our results, NC group (negative control) was biocompatible because the inflammatory process was non-significant at all periods and the empty tube allowed for the formation of connective tissue, as in other publications [22,24,26-28].
The zinc oxide eugenol used as Positive Control (PC group) is an inflammatory material because when in contact with tissue, it has been associated with areas of tissue necrosis [22,24], as was seen in our results. Analyzing the experimental medications and comparing them with control groups, it was possible to identify the absence of biocompatibility of them. The data of both medications were like the PC group and statistically different from the NC group. Investigating CH and Aloe vera independently, it was plausible to recognize the biocompatibility of both substances. Although CH induces an initial inflammatory reaction when in contact with tissue, this disappears and tissue repair occurs [21]. Moreover, in cell culture with fibroblasts or osteoblasts, the CH is no cytotoxic [29]. Aloe vera is an anti-inflammatory substance [16] and permits cell proliferation and healing [13-15,25,30]. Also, with neutral pH (6.6±0.5), Aloe vera is no-irritating to tissues [31] and in therapeutic doses, it does not present toxicological reactions [15]. Considering the biocompatibility of CH and Aloe vera, we could expect the biocompatibility of experimental medications used in this study. However, it was not verified. One explanation for the irritation observed in the experimental medications can be due to the high dosage of Aloe vera [15,31].
Despite the concentration of gel present in experimental paste 1 being compatible with a therapeutic dose, the quantity of gel in the formulation can result in an irritant reaction. In experimental paste 2, the use of concentrated and undiluted powder can be responsible for high dosage. Also, the irritability of the experimental medications can be attributed to the incompatibility between their components. The lack of acid-base balance, ionization reactions, the aqueous solubility of components and/or severe changes in pH can promote the incompatibility between substances [32], contributing to the irritability of drugs or medications.
Conclusion
The experimental medications were not biocompatible with the tissues, and new formulations with changes of components and their concentration must be performed to justify the use of Aloe vera in calcium hydroxide pastes.
Author Contributions
Conceptualization: Mori GG. Data curation: Mori GG, Jacomoni LM. Formal analysis: Mori GG. Funding acquisition: Mori GG, Jacomini LM Investigation: Mori GG, Santinoni CS, Jacomini LM. Methodology: Mori GG, Santinoni CS, Jacomini LM. Project administration: Mori GG. Resources: Mori GG, Jacomini LM. Supervision: Mori GG. Writing - original draft: Mori GG, Santinoni CS, Jacomini LM. Writing-review and editing: Mori GG, Santinoni CS.
Funding
This work was supported by São Paulo Research Foundation- FAPESP, Brazil (2012/15922-8).
References
- Mohammadi Z, Dummer PMH (2011) Properties and applications of calcium hydroxide in endodontics and dental traumatology. Inter Endod J 44(8): 697-730.
- Jahromi MZ, Motamedi MRK (2019) Effect of calcium hydroxide on inflammatory root resorption and ankylosis in replanted teeth compared with other intracanal materials: A review. Restor Dent Endod 44(3): e32.
- Mori GG, Andrade BS, Araujo MB (2020) Endodontic approach in a replanted tooth with an immature root apex and chronic apical periodontitis: A case report. Restor Dent Endod 45(3): e29.
- Tronstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I (1981) pH changes in dental tissues after root canal filling with calcium hydroxide. J Endod 7(1): 17-21.
- Farhadian N, Godiny M, Mansouri A, Moradi S, Tajehmiri A, et al. (2020) Hydrophilic natural polymers for sustained-controlled release of calcium hydroxide. Iran J Pharm Res 19(2): 323-332.
- Sharma G, Ahmed HMA, Zilm PS, Rossi-Fedele G (2018) Antimicrobial properties of calcium hydroxide dressing when used for long-term application: A systematic review. Aust. Endod J 44(1): 60-65.
- Arruda MEF, Neves MAS, Diogenes A, Mdala I, Guilherme BPS, et al. (2018) Infection control in teeth with apical periodontitis using a triple antibiotic solution or calcium hydroxide with chlorhexidine: A randomized clinical trial. J Endod 44(10): 1474-1479.
- Bedran NR, Nadelman P, Magno MB, Neves AA, Ferreira DM, et al. (2020) Does calcium hydroxide reduce endotoxins in infected root canals? Systematic review and meta-analysis. J Endod 46(11): 1545-1558.
- Youssef AR, Emara R, Taher MM, Al-Allaf FA, Almalki M, et al. (2019) Effects of mineral trioxide aggregate, calcium hydroxide, bio dentine and Emdogain on osteogenesis, odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral Health 19(1): 133.
- Mori GG, Ferreira FC, Batista FR, Godoy AM, Nunes DC (2009) Evaluation of the diffusion capacity of calcium hydroxide pastes through the dentinal tubules. Braz Oral Res 23(2): 113-118.
- Montero JC, Mori GG (2012) Assessment of ion diffusion from a calcium hydroxide-propolis paste through dentin. Braz Oral Res 26(4): 318-322.
- Farhad AR, Barekatain B, Allameh M, Narimani T (2012) Evaluation of the antibacterial effect of calcium hydroxide in combination with three different vehicles: An in vitro Dent Res J 9(2): 167-172.
- Jettanacheawchankit S, Sasithanasate S, Sangvanich P, Banlunara W, Thunyakitpisal P (2009) Acemannan stimulates gingival fibroblast proliferation, expressions of keratinocyte growth factor-1, vascular endothelial growth factor and type I collagen and wound healing. J Pharmacol Sci 109(4): 525-531.
- Jittapiromsak N, Sahawat D, Banlunara W, Sangvanich P, Thunyakitpisal P (2010) Acemannan, an extracted product from Aloe vera stimulates dental pulp cell proliferation, differentiation, mineralization and dentin formation. Tissue Eng Part A 16: 1997-2006.
- Chantarawaratit P, Sangvanich P, Banlunara W, Soontornvipart K, Thunyakitpisal P (2014) Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model. J Periodontal Res 49(2): 164-178.
- Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP (2020) Pharmacological update properties of aloe vera and its major active constituents. Molecules 25(6): 1324.
- Ghasemi N, Behnezhad M, Asgharzadeh M, Zeinalzadeh E, Kafil HS (2020) Antibacterial properties of aloe vera on intracanal medicaments against enterococcus faecalis biofilm at different stages of development. In J Dent 28: 8855277.
- Li CY, Suzuki K, Hung YL, Yang MS, Yu CP, et al. (2017) Aloe metabolites prevent LPS-induced sepsis and inflammatory response by inhibiting mitogen-activated protein kinase activation. Am J Chin Med 45(4): 847-886.
- Liu C, Cui Y, Pi F, Cheng Y, Guo Y, et al. (2019) Extraction, purification, structural characteristics, biological activities and pharmacological applications of Acemannan, a polysaccharide from Aloe vera: A Review. Molecules 24(8): 1554.
- Batista VE, Olian DD, Mori GG (2014) Diffusion of hydroxyl ions from calcium hydroxide and Aloe vera pastes. Braz Dent J 25(3): 212-216.
- Hauman CH, Love RM (2003) Biocompatibility of dental materials used in contemporary endodontic therapy: A review. Part 1. Intracanal drugs and substances. Int Endod J 36(2): 75-85.
- Mori GG, Rodrigues SS, Shibayama ST, Pomini M, Amaral COF (2014a) Biocompatibility of a calcium hydroxide-propolis experimental paste in rat subcutaneous tissue. Braz Dent J 25(2): 104-108.
- Jamovi (2021) The Jamovi project (Version 1.6)-Computer software: Jamovi.
- Mori GG, Teixeira LM, Oliveira DL, Jacomini LM, Silva SR (2014b) Biocompatibility evaluation of Bio dentine in subcutaneous tissue of rats. J Endod 40(9): 1485-1488.
- Nai GA, Logar GA, Mori GG, Teixeira LM, Silva BCF, et al. (2016) Evaluation of the genotoxicity and mutagenicity of Ca3SiO5-based cement. Braz Oral Res 30(1): S1806-83242016000100277.
- Cintra LTAC, Benetti F, Queiroz IOA, Lopes JMA, Oliveira SHP, et al. (2017) Cytotoxicity, biocompatibility and biomineralization of the new high-plasticity MTA material. J Endod 43(5): 774-778.
- Silva ECA, Tanomaru-Filho M, SIlva GF, Delfino MM, Cerri PS, et al. (2020) Biocompatibility and bioactive potential of new calcium silicate-based endodontic sealers: Bio-C sealer and sealer plus BC. J Endod 46(10): 1470-1477.
- Hosinho RA, Delfino MM, Da Silva GF, Guerreiro-Tanumaru JM, Tanumaru-Filho M, et al. (2021) Biocompatibility and bioactive potential of the NeoMTA plus endodontic bio ceramic-based sealer. Restor Dent Endod 46(1): e4.
- Gonçalves GSY, Gregorio D, Custodio IR, Maia LP, Piazza B, et al. (2021) Cytotoxicity and osteogenic potential of experimental medication with calcium hydroxide and activated charcoal. Research, Society and Development 10(5): e26010514671.
- Moriyama M, Moriyama H, Uda J, Kubo H, Nakajima Y, et al. (2016) Beneficial effects of the genus Aloe on wound healing, cell proliferation and differentiation of epidermal keratinocytes. Plos One 11(10): e0164799.
- Vogler BK, Ernst E (1999) Aloe vera: A systematic review of its clinical effectiveness. Br J Gen Pract 49(447): 823-828.
- Newton DV (2009) Drug incompatibility chemistry. Am J Health-Syst Pharm 66(4): 348-357.
© 2025 Carolina Santinoni. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






