- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Biological Control of Fusarium Wilt in Tomato Using Trichoderma Harzianum and T. Viride: In Vitro and Greenhouse Evaluation
Ioannis Vagelas*
Department of Agriculture, University of Thessaly, Laboratory of Plant Pathology, Crop Production and Rural Environment, School of Agricultural Sciences, Greece
*Corresponding author:Ioannis Vagelas, Department of Agriculture, University of Thessaly, Laboratory of Plant Pathology, Crop Production and Rural Environment, School of Agricultural Sciences, Greece
Submission: October 14, 2025;Published: November 17, 2025

ISSN 2637-7659Volume15 Issue 4
Abstract
Trichoderma harzianum and T. viride were evaluated for their antagonistic activity against Fusarium oxysporum f. sp. lycopersici, the causal agent of tomato wilt, through dual-culture assays and greenhouse experiments. Both species significantly inhibited the radial growth of the pathogen in vitro, with T. harzianum exhibiting the highest suppression. In greenhouse trials, soil application of conidial suspensions of Trichoderma spp. led to a significant reduction in disease severity and a marked increase in stem fresh weight compared to the infected control. Among the two, T. harzianum demonstrated superior efficacy in promoting plant growth and suppressing wilt symptoms. These findings support the potential of Trichoderma spp. as effective biological control agents for integrated management of Fusarium wilt in tomato cultivation.
Keywords:Biological control; Soil-borne pathogens; Antagonistic fungi; Plant growth promotion
Introduction
Soil-borne fungal pathogens such as Fusarium oxysporum f. sp. lycopersici (Fol) represent a persistent threat to tomato cultivation worldwide, causing significant yield losses and limiting sustainable production. Traditional chemical control methods, while effective in the short term, often lead to environmental concerns, pathogen resistance and disruption of soil microbiota. As a result, the development of eco-friendly and sustainable alternatives has become a central focus in plant pathology and integrated pest management. Among the most promising biological control agents are species of the genus Trichoderma, which exhibit multiple mechanisms of action including mycoparasitism, competition for nutrients and space, production of anti-fungal metabolites, and induction of systemic resistance in host plants [1]. Recent studies have demonstrated that BCAs such as Trichoderma harzianum and T. viride not only suppress Fol in vitro but also enhance plant vigor and yield under greenhouse and field conditions [2,3]. Furthermore, Trichoderma spp. has been shown to interact synergistically with plant defense pathways, priming host responses and modulating hormonal signaling cascades that contribute to long-term resistance [4]. These multifaceted interactions underscore the potential of Trichoderma as both a biocontrol agent and a biofertilizer, aligning with the goals of sustainable agriculture. This study aims to evaluate the antagonistic activity of T. harzianum and T. viride against Fol through dual-culture assays and greenhouse experiments. By focusing on these two species, we seek to clarify their comparative efficacy and practical applicability in tomato wilt management under controlled conditions.
Material and Methods
Pathogen and antagonist cultures
Fusarium oxysporum f. sp. lycopersici (IMI 141140) and Rhizoctonia solani (IMI 208019) were used as model phytopathogenic fungi. Native Trichoderma isolates were recovered from soil samples collected in tomato cultivation areas in Greece. Isolation was performed using serial dilution and plating on Rose Bengal Agar (RBA), followed by subculturing on Potato Dextrose Agar (PDA). Morphological identification was based on colony characteristics, conidiophore branching, phialide arrangement and conidial morphology, following the taxonomic criteria described by Rahman et al. [5]. To confirm species identity and assess phenotypic consistency, the native isolates were compared with authenticated strains T. harzianum (IMI 392433) and T. viride (IMI 076304). Comparative analysis focused on conidiophore architecture, conidial dimensions, pigmentation and colony growth patterns.
In vitro antagonism assay
Dual-culture assays were performed following the method of Royse [6]. Mycelial plugs (5mm diameter) of the pathogen Fol and the BCA were placed equidistantly on Petri dishes containing either Potato Dextrose Agar (PDA) or V8 juice-based Agar (V8A). Plates were incubated at 25±1 °C for 144 hours [7]. Radial growth inhibition of the pathogen was measured and expressed as a percentage reduction compared to control plates. To confirm BCA antagonist activities, R. solani were also used.
Greenhouse experiment
To evaluate the biocontrol efficacy of T. harzianum and T. viride against Fol, tomato seeds (Solanum lycopersicum cv. Marmande, susceptible to Fol) were sown in autoclaved vermiculite: Peat (1:1 v/v) and maintained under controlled conditions (24±2 °C, 70- 80% RH, 16-h photoperiod) for 20 days. At the 2-4 true-leaf stage, seedlings were transplanted into pots containing a sterilized soil mixture (peat: loam: sand, 3:1:1 v/v). Pathogen inoculation was performed immediately after transplanting by applying 10ml of a Fol micro conidial suspension (1×106 microconidia mL-1) directly to the rhizosphere [8]. Biocontrol Agents (BCAs) were applied simultaneously by drenching the soil with 5ml of Trichoderma conidial suspension (1×10⁶ conidia mL-1) per pot. No further applications were made during the experimental period. This single co-application design was chosen to simulate practical field conditions and assess the immediate antagonistic and protective effects of Trichoderma spp. under pathogen pressure [9]. Control treatments included: (i) infected control (Fol only) and (ii) noninoculated control (no Fol, no BCA). Plants were maintained in a greenhouse at 22±3 °C for 30 days. Disease severity was assessed using a standardized wilt index 1-5 scale: 1, (0-24%, healthy plant, all leaves green; 2, (25-49%) lower leaves yellow; 3, (50-74%) lower leaves dead and some upper leaves wilted; 4, (75-90%) lower leaves dead and upper leaves wilted; 5, (100%) dead plant. Further, all plants were placed in humid chambers, and the presence or absence of the pathogen in the crown was recorded after 48h incubation [10]. Stem fresh weight and stunting incidence were also recorded.
Data collection and statistical analysis
All experimental data were subjected to statistical analysis using JASP 0.18 (open-source statistical software). For in vitro assays, percentage inhibition values were analyzed using one-way Analysis of Variance (ANOVA), followed by Tukey’s multiple range test to determine significant differences among treatments (P<0.05). Greenhouse data-including disease severity, disease incidence, pathogen isolation, and stem fresh weight-were analyzed using factorial ANOVA. Percentage data (disease incidence and pathogen isolation) were arcsine square-root transformed prior to analysis to normalize variance. Stem fresh weight data were analyzed using parametric ANOVA, with homogeneity of variance confirmed via Levene’s test. Significant differences among treatments were determined using Tukey’s HSD test at P=0.05.
Result
In vitro antagonism assay
Simultaneous growth of phytopathogens with Trichoderma species on agar plates revealed rapid colonization of the medium, aggressive expansion over unoccupied surfaces, and marked inhibition of pathogen development (Table 1). Specifically, T. harzianum exhibited vigorous growth over Fol mycelia, with observable hyphal coiling and parallel progression (Figure 1). On V8 medium, its interaction with R. solani was less aggressive compared to T. viride but still suppressive (Table 1). Similarly, T. viride formed a distinct barrier against both pathogens, followed by dense green conidiation across the entire surface of the medium (Figure 2). A characteristic coconut-like odor was noted during incubation, consistent with secondary metabolite production. In both cases, a diffused light green zone was observed around the antagonist colonies, suggestive of fungi toxin secretion-possibly linked to known Trichoderma-derived metabolites such as peptaibols or viridin-like compounds.
Table 1:Mean % Inhibition of Radial growth of Fol and R. solani (Rs) after 144 h incubation at 25 °C.

*SEM (Standard Error of Mean). **Values within a column followed by the same letter do not differ significantly according to Tukey’s test (P=0.05). Values are based on 12 replicates per treatment.
Figure 1:Microscopic interaction between Trichoderma harzianum and Fusarium oxysporum f. sp. lycopersici. Hyphae of T. harzianum exhibit coiling and parallel growth around the pathogen’s hyphae. Magnification: ×400.

Figure 2:Dual culture of Trichoderma viride and Fusarium oxysporum f. sp. lycopersici on PDA (left) and V8 (right) medium. T. viride rapidly colonized the plate, forming a dense green conidial layer and establishing a physical barrier at the edge of the F. oxysporum colony.

Greenhouse experiment
Application of T. harzianum and T. viride significantly influenced disease parameters and plant growth under greenhouse conditions (Table 2). Disease severity was markedly reduced in both treatments compared to the infected control, with T. harzianum showing the lowest severity score (28.4%) and T. viride following closely (35.7%). These values were significantly lower than the infected control (68.9%) and comparable to the non-inoculated control (14.2%) (Table 2). Disease incidence followed a similar trend, with T. harzianum and T. viride reducing symptomatic plants to 42.5% and 47.5%, respectively, in contrast to 92.5% in the infected control. Pathogen isolation from crown tissues confirmed the suppressive effect of both Trichoderma species, with isolation rates of 40.0% (T. harzianum) and 45.0% (T. viride), significantly lower than the infected control (90.0%) (Table 2). Tomato stem fresh weight was significantly enhanced in all BCA treatments. Plants treated with T. harzianum reached a mean fresh weight of 12.8g, statistically higher than the infected control (6.2g) and comparable to the non-inoculated control (13.5g). T. viride also improved plant vigor (10.9g), though to a lesser extent (Table 2). Statistical analysis confirmed significant differences among treatments for all parameters except pathogen isolation, which showed marginal significance (Table 2).
Table 2:Effect of Trichoderma spp. on Fusarium wilt parameters under greenhouse conditions.
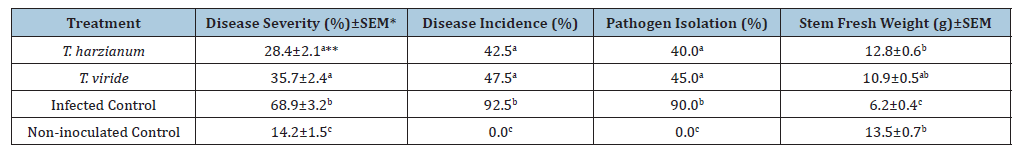
*SEM: Standard Error of Mean, based on 10 replicates per treatment. **Means followed by the same letter within a column do not differ significantly according to Tukey’s test (P=0.05).
Conclusion
This study provides robust evidence for the antagonistic efficacy of T. harzianum and T. viride against two major soil-borne pathogens of tomato: F. oxysporum f. sp. lycopersici and R. solani. In vitro dual-culture assays revealed significant inhibition of radial growth for both pathogens, with T. harzianum exhibiting superior suppression of Fol and T. viride demonstrating strong inhibition of R. solani, particularly on V8 medium. Rashid et al. [11] showed that both T. harzianum and T. viride exhibit antagonistic properties against F. oxysporum and F. solani through mechanisms such as competition for nutrients and space, mycoparasitism and antibiosis. T. harzianum achieved suppression levels comparable to the non-inoculated control, while T. viride formed a dense conidial barrier and produced volatile compounds suggestive of fungitoxic activity. These findings align with our results, highlighting the effectiveness of these Trichoderma species in suppressing the pathogen. Greenhouse trials confirmed the biocontrol potential of both species, as soil application of conidial suspensions led to reduced disease severity and incidence, lower pathogen isolation rates and enhanced stem fresh weight. Similar results were reported by Srivastava et al. [12], who found that Trichoderma spp. enhances plant defense mechanisms by increasing peroxidase and polyphenol oxidase activities, thereby improving resistance to Fusarium wilt.
Our results (in vitro and greenhouse experiments) are like those of Jamil A. [13], who reported that the application of Trichoderma spp. against Fol resulted in the lowest disease severity, as well as the highest levels of physiological activity, biochemical and antioxidant contents and overall improvements in plant growth and yield during field trials. Overall, the integration of Trichoderma spp. into tomato cultivation systems offers a sustainable alternative to chemical fungicides, especially in Mediterranean and organic production contexts. Future research should focus on formulation stability, field-scale validation and molecular profiling of induced resistance pathways to optimize application strategies and longterm effectiveness.
References
- Awad Allah EF, Shams AH, Helaly AA, Ragheb EI (2022) Effective applications of Trichoderma spp. as biofertilizers and biocontrol agents mitigate tomato fusarium wilt disease. Agriculture 12(11): 1950.
- Sehim AE, Hewedy OA, Altammar KA, Alhumaidi MS, Abd Elghaffar RY (2023) Trichoderma asperellum empowers tomato plants and suppresses Fusarium oxysporum through priming responses. Frontiers in Microbiology 14: 1140378.
- Sorahinobar M, Eslami S, Shahbazi S, Najafi J (2025) A mutant Trichoderma harzianum improves tomato growth and defense against Fusarium European Journal of Plant Pathology 172: 169-184.
- Meddad Hamza A, Benzina F, Meddad C, Hamza N, Reghmit A, et al. (2023) Biological control of arbuscular mycorrhizal fungi and Trichoderma harzianum against Fusarium oxysporum and Verticillium dahliae induced wilt in tomato plants. Egyptian Journal of Biological Pest Control 33: 1-10.
- Rahman A, Begum MF, Rahman M, Bari MA (2011) Isolation and identification of Trichoderma species from different habitats and their use for bioconversion of solid waste. Turkish Journal of Biology 35(2): 183-194.
- Royse DJ (1978) The influence of fungi isolated from peach twigs on the pathogenicity of Cytospora cincta. Phytopathology 68: 603.
- Selva Amala A, Parthiban VK, Sudha A, Gopalakrishnan C, Swarnakumari N, et al. (2024) Antifungal and plant-growth promoting potency of Trichoderma asperellum against Fusarium wilt on tomato. Journal of Plant Pathology.
- Vagelas IK (2002) Efficacy of Pseudomonas oryzihabitans as a biocontrol agent of root pathogens (PhD thesis). University of Reading, School of Agriculture, Policy and Development, UK.
- Lewis JA, Papavizas GC (1984) A new approach to stimulate population proliferation of Trichoderma species and other potential biocontrol fungi introduced into natural soils. Phytopathology 74: 1240-1244.
- De Cal AD, PascualS, Melgarejo P (1997) Infectivity of chlamydospores vs microconidia of fusarium oxysporum f.sp. lycopersici on tomato. Journal of Phytopathology 145(5-6): 231-233.
- Rashid TS, Qadir SA, Awla HK (2021) Induction of defense related enzymes and biocontrol efficacy of Trichoderma harzianum in tomato plants infected with Fusarium oxysporum and Fusarium solani. Acta Agriculturae Slovenica 117: 1.
- Srivastava R, Khalid AN, Singh US, Sharma A (2010) Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biological Control 53(1): 24-31.
- Jamil A (2021) Antifungal and plant growth promoting activity of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici colonizing tomato. Journal of Plant Protection Research 61(3): 243-253.
© 2025 Ioannis Vagelas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






