- Submissions

Full Text
Modern Approaches in Drug Designing
From In Vitro to In Vivo: Groundbreaking Pathways in Ocular Biomaterials Testing
Andreza Maria Ribeiro1*, Thais HS Flores-Sahaguns2 and Fabio Furtado2
1 Department of Engineering and Material Sciences (PIPE), University of Federal of Parana (UFPR), Brazil
2 Department of Mechanical Engineering, University of Federal of Parana (UFPR), Brazil
*Corresponding author:Andreza Maria Ribeiro, Department of Engineering and Material Sciences (PIPE), University of Federal of Parana (UFPR), Brazil
Submission: November 27, 2025;Published: December 12, 2025

ISSN: 2576-9170 Volume5 Issue 1
Abstract
The development of biomaterials for ocular applications has advanced considerably, driven by the need for safe and effective devices such as intraocular lenses, corneal scaffolds, and contact lenses. A critical step in this process is the biological evaluation of materials, which ensures biocompatibility and predicts clinical performance. In vitro assays, including cytotoxicity tests such as MTT and MTS, provide essential insights into cell viability and material interactions with ocular tissues. Complementary methods, such as the HET-CAM assay, offer ethical and efficient alternatives to traditional animal testing by assessing irritation potential in a vascularized membrane model. In vivo studies remain indispensable for evaluating longterm stability, optical clarity, and tissue integration, although their use is increasingly complemented by advanced in vitro and tissue-engineered models. Emerging approaches, including alternative assays such as HET-CAM, smart polymers responsive to ocular microenvironments, nanocomposite scaffolds, and microfluidic ocular-on-chip systems, represent innovative strategies to accelerate the safe translation of biomaterials into ophthalmic practice. This mini review discusses conventional and alternative assays for ocular biomaterials, including recent sensitive in vitro protocols designed to evaluate acute irritation and phototoxicity of medical devices and ophthalmic drugs, as well as organ-on-chip platforms that recreate dynamic ocular environments for predictive testing. Together, these approaches form a comprehensive framework for the characterization of ocular biomaterials, supporting innovation while reducing reliance on animal experimentation.
Keywords:Ocular biomaterials; Biocompatibility; Cytotoxicity assays; Het-cam; In vivo evaluation; Smart polymers; Epioculatm; Microfluidic ocular-on-chip
Introduction
Biomaterials are materials designed to interface with biological systems to evaluate, treat, enhance, or replace tissues, organs, or physiological functions. According to Hoffman (2004), biomaterials can be classified into four major categories: polymers, metals, ceramics, and composites. Within this context, biological evaluation assays represent an essential tool during the development and characterization of these materials, principally for biocompatibility [1]. Biocompatibility does not only encompass the potential effects of biomaterials on the host organism, but also the reciprocal influence of the physiological environment on the material itself. Both natural and synthetic biomaterials exhibit intrinsic properties that determine their stability and compatibility. These characteristics must be thoroughly understood to predict the clinical performance of biomaterials such as ocular devices throughout their application period [1,2]. In the field of ophthalmology, the demand for safe and effective biomaterials has grown significantly, driven by applications such as intraocular lenses, corneal scaffolds, and contact lenses [3]. Biological evaluation assays are therefore indispensable tools in the development and characterization of ocular biomaterials. In vitro cytotoxicity tests, standardized by ISO 10993-5, represent the first step in screening materials for safety, while alternative models such as HET- CAM, sensitive in vitro protocols for ocular biocompatibility testing, highlight the importance of replacing traditional animal models with standardized assays capable of predicting acute irritation and phototoxicity, and tissueengineered corneal equivalents provide ethical and predictive approaches that reduce reliance on animal testing [4-6]. Ultimately, the integration of in vitro, alternative tests, and in vivo assays forms a comprehensive framework for advancing innovative biomaterials toward clinical translation in ophthalmology. The minireview aims to support future engineers and pharmaceutical scientists to drive in development of standardized in vitro testing protocols. By integrating biological assays with mechanical characterization, the optimization of biomaterials, including tissue scaffolds, polymeric implants, polymeric devices, and ceramics, can be achieved. This approach enhances the efficiency, quality, and safety of biomaterials in in vivo configurations for future biomedical applications.
Innovative Biomaterials
Recent developments focus on smart biomaterials that can respond to stimuli such as pH, temperature, or light, enabling the controlled release of drugs within ocular tissues [7,8]. Nextgeneration biomaterials are designed with dual functionality, combining structural support with therapeutic delivery [9,10]. Nanocomposite scaffolds and biofunctionalized hydrogels represent promising strategies for enhancing corneal regeneration, improving intraocular lens performance, and facilitating retinal repair [2,11].
Biological Assays for Ocular Biomaterials
Biological assays for the evaluation of biomaterial biocompatibility can be conducted through a wide range of in vitro and in vivo tests. These assays provide valuable insights into the interaction between the material and the physiological environment, as well as the potential risks associated with its clinical application [4,12]. Such evaluations are crucial to identify biomaterials that lack the necessary characteristics for safe use in ocular studies [13]. Cytotoxicity assays performed in vitro should be considered the initial step in biocompatibility testing [14-16]. These tests allow the identification of materials exhibiting cytotoxic behavior, thereby ensuring that only the most suitable candidates progress to in vivo evaluation. This strategy not only minimizes the number of experimental animals required but also reduces time and resources, aligning with ethical and regulatory principles in biomedical research. For ocular applications, biological evaluation of biomaterials requires specific essays that reflect the unique physiology of the eye [9]. In vitro cytotoxicity tests remain the first step, but ocular biomaterials demand additional evaluations such as epithelial cell adhesion, corneal transparency maintenance, and inflammatory response assays [16]. These tests provide critical insights into how polymers, hydrogels, or composite scaffolds interact with delicate ocular tissues. Subsequent in vivo studies often employ animal models to assess intraocular tolerance, biodegradation, and long-term stability. For example, hydrogelbased corneal implants and intraocular lenses must demonstrate not only the absence of cytotoxicity but also optical clarity and minimal induction of neovascularization [17]. Such assays are indispensable to predict the clinical performance of biomaterials before translation into ophthalmic devices.
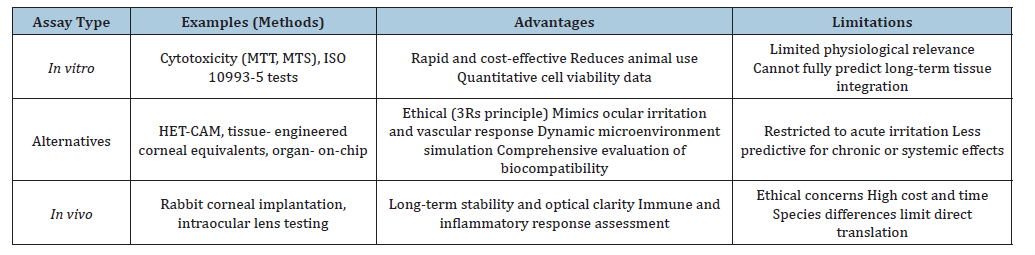
In Vitro Cytotoxicity Testing According to ISO 10993-5
The ISO 10993-5 standard (2009) establishes three categories of in vitro cytotoxicity assays: Extract testing, direct contact testing, and indirect contact testing [4]. Extract and direct contact assays enable both qualitative and quantitative evaluation of cytotoxicity, as well as the assessment of cell viability when exposed to biomaterials. In contrast, the indirect contact assay provides only qualitative information regarding cytotoxic effects. These methodologies are fundamental in the early stages of biomaterial evaluation, ensuring that ocular devices are screened for safety before advancing to in vivo studies [11,18].
Quantitative Assessment of Cell Viability
Quantitative evaluation of cell viability can be performed using colorimetric methods with dyes such as MTT 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide) and MTS (3- (4,5- dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4- sulfophenyl)-2H-tetrazolium). MTT is a yellow salt reduced by mitochondrial dehydrogenase activity, resulting in the formation of purple formazan crystals. This reduction occurs exclusively in living cells, allowing viability to be determined by the intensity of the purple coloration, which is proportional to the amount of formazan produced. A limitation of MTT is that the insoluble formazan crystals require organic solvents such as DMSO or isopropanol for dissolution [19-21]. MTS, in contrast, is reduced by the same enzymatic activity but yields water-soluble formazan products. This eliminates the need for organic solvents, making the assay more straightforward. MTS is typically used in combination with an electron coupling agent, PMS (Phenazine Metho Sulfate), to enhance the reaction [19,20]. These assays provide an efficient pathway toward in vivo testing by pre-selecting suitable biomaterials, thereby reducing the number of animal experiments, time, and resources required.
Alternative Tests in Ocular Biomaterial: Het-Cam Assay, Sensitive In Vitro Protocols, Microfluidic Organ-On-Chip Models
The Hen’s Egg Test on the Chorioallantois Membrane (HETCAM) is an in vitro method widely recognized as an alternative to traditional animal-based ocular irritation tests, such as the Draize Eye Test. It employs the highly vascularized chorioallantois membrane of fertilized hen’s eggs, which responds to chemical injury with inflammation, hemorrhage, or coagulation. These reactions closely mimic the conjunctival tissue response in the human eye [22,23]. Advantages of HET-CAM testing: i. Ethical compliance: Supports the 3Rs principle (replace, reduce, refining animal use), minimizing reliance on live animal models; ii. Sensitivity: The CAM membrane is highly vascularized, making it an effective surrogate for mucosal irritation: iii. Efficiency: Provides rapid results for screening ocular biomaterials, formulations, or surface modifications; iv. Applicability: Frequently used in cosmetics, pharmaceuticals, and biomaterials research to assess acute irritation potential Limitations: i. The CAM model does not fully replicate the complexity of mammalian ocular physiology; ii. Results are most reliable for acute irritation but less predictive for chronic or long-term biocompatibility. The relevance for ocular biomaterials, in the context of biomaterials for ophthalmic applications, such as hydrogels, intraocular lenses, or corneal scaffolds, the HET-CAM assay serves as a preliminary screening tool. It allows researchers to identify materials with low irritation potential before advancing to in vivo ocular models [5,24,25]. This approach reduces experimental costs and aligns with ethical standards in biomedical research. The sensitive in vitro protocols recently developed for ocular biocompatibility testing employ reconstructed human cornea-like tissue (EpiOcularTM) to assess acute irritation and phototoxicity of medical devices and ophthalmic drugs [26]. These assays combine tissue viability measurements with inflammatory markers such as IL-1α release, providing a standardized and ethically acceptable alternative to traditional rabbit eye tests. Importantly, they expand the applicability of ISO 10993-23 and OECD TG 498 guidelines, supporting more predictive and reproducible evaluation strategies for ophthalmic products [26-28].
In contrast, microfluidic organ-on-chip models represent a complementary and more advanced approach. Ocular-on-chip systems integrate human corneal or retinal cells within perfused microchannels, recreating dynamic physiological conditions such as nutrient flow, mechanical stress, and cellular communication. This enables real-time monitoring of biomaterial interactions, drug release kinetics, and long-term tissue responses. By bridging the gap between conventional in vitro assays and clinical applications, organ-on-chip platforms provide highly predictive insights while reducing reliance on animal experimentation [29-31]. Microfluidic ocular-on-chip platforms have already been applied to the testing of diverse ophthalmic products. For example, eye-on-chip systems have been used to assess the irritation and toxicity of ophthalmic solutions and eye drops, simulating tear flow and repeated exposure under physiological conditions [29]. Similarly, contact lenses have been evaluated for their long- term interaction with corneal epithelial cells, including hydration, permeability, and therapeutic agent release [30]. In addition, corneal scaffolds and regenerative biomaterials have been tested for cell adhesion, inflammatory response, and tissue integration, while drug delivery systems such as nanoparticles and sustained-release implants have been studied for their release kinetics in dynamic ocular environments [32]. Together, these applications demonstrate the predictive power of ocular-on-chip models as ethical and translational alternatives to animal testing in ophthalmic biomaterials research.
In Vivo Assays for Ocular Biomaterials
Following in vitro cytotoxicity screening, in vivo assays are essential to validate the safety and performance of biomaterials in ocular environments [33]. Animal models, such as rabbits and rodents, are frequently employed to assess intraocular tolerance, biodegradation, and long-term stability of implants [34]. These studies provide critical information on inflammatory responses, neovascularization, and tissue integration, which cannot be fully replicated in in vitro systems. For corneal applications, in vivo assays evaluate transparency, epithelial regeneration, and stromal integration of hydrogel-based implants or bioengineered scaffolds (Table 1). Intraocular lenses and polymeric devices are tested for optical clarity, mechanical stability, and absence of adverse immune reactions. Such evaluations are indispensable to predict clinical outcomes and guide the translation of biomaterials into ophthalmic practice [17,35] (Table 2).
Table 1:Examples of ocular biomaterials and biological assays [3,17,36,37].
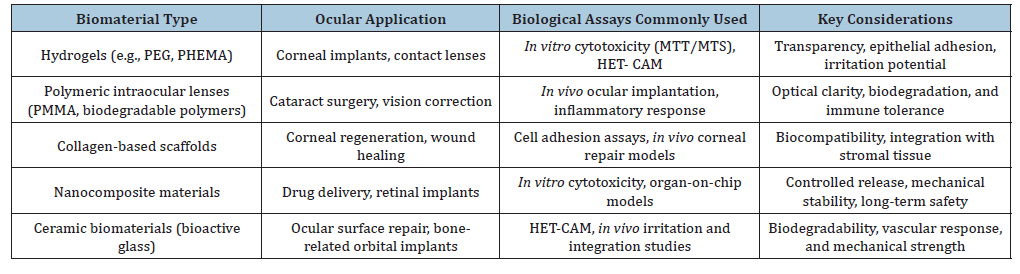
Table 2:Biological assays for ocular biomaterials: Advantages and limitations [4,5,17,22,31,38].
Conclusion
The evaluation of biomaterials for ocular applications requires a comprehensive framework that integrates in vitro, alternative, and in vivo assays. Cytotoxicity tests such as MTT and MTS provide essential insights into cell viability, while innovative approaches like HET-CAM, tissue-engineered corneal equivalents, and organon- chip models offer ethical and predictive alternatives to animal testing. Recent sensitive in vitro protocols employing reconstructed human cornea-like tissue (EpiOcularTM) have demonstrated the ability to reliably assess acute irritation and phototoxicity, expanding the applicability of ISO 10993-23 and OECD TG 498 guidelines. In parallel, microfluidic ocular-on-chip systems recreate dynamic physiological environments, enabling real-time evaluation of biomaterial interactions, drug release kinetics, and long-term tissue responses, thereby bridging the gap between laboratory assays and clinical translation [36-38]. Despite the indispensable role of in vivo studies in assessing long-term stability and optical performance, the future of ocular biomaterials lies in the development of smart, multifunctional materials capable of combining structural support with therapeutic delivery. By embracing advanced biological models such as EpiOcularTM protocols and ocular-on-chip platforms, alongside sustainable innovation, researchers can accelerate the translation of biomaterials into safe and effective ophthalmic devices, ultimately improving patient outcomes and reducing reliance on animal experimentation.
Acknowledgment
This work was supported by the Brazilian National Research Council, CNPq, Brazil. The authors wish to thank the National Council for Scientific and Technological Research (CNPq, fellowships process numbers 249251/2013-2 and 302459/2023-5).
References
- Hoffman AS (1992) Present and emerging applications of polymeric biomaterials. Clinical Materials 11(1-4): 13-18.
- Meek KM, Knupp C (2015) Corneal structure and transparency. Progress in Retinal and Eye Research 49: 1-16.
- Ferraz MP (2022) Biomaterials for ophthalmic applications. Applied Sciences 12(12):
- ISO (2009) ISO 10993-5: 2009 Biological evaluation of medical devices-part 5: Tests for in vitro 3rd (edn), International Organization for Standardization Geneva, Switzerland, pp. 34.
- Eder C, Falkner E, Mickel M, Grisar CC, Appl H, et al. (2005) A modified HET-CAM approach for biocompatibility testing of medical devices. Animal Welfare 14(4): 297-302.
- Bos G (2022) ISO 13485: 2016: Medical devices-quality management systems-requirements for regulatory purposes, in medical regulatory affairs. (3rd edn), Jenny Stanford Publishing, Singapore, pp. 21.
- Ribeiro AM, Figueiras A, Veiga F (2015) Improvements in topical ocular drug delivery systems: Hydrogels and contact l Journal of Pharmacy & Pharmaceutical Sciences 18(5): 683.
- Gupta, P, Vermani K, Garg S (2002) Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discovery Today 7(10): 569-579.
- Ribeiro AM, Neumann IA (2017) Advances in composite hydrogels for ocular drug delivery and biomedical engineering application, in functional hydrogels in drug delivery. CRC Press, Florida, USA, pp. 303-326.
- Ribeiro A, Veiga F, Santos D, Labandeira JJT, Concheiro A, et al. (2011) Bioinspired imprinted PHEMA-hydrogels for ocular delivery of carbonic anhydrase inhibitor d Biomacromolecules 12(3): 701-709.
- Lloyd AW, Dropcova S, Faragher RG, Gard PR, Hanlon GW, et al. (1999) The development of in vitro biocompatibility tests for the evaluation of intraocular biomaterials. Journal of Materials Science: Materials in Medicine 10(10): 621-627.
- Ratner BD, Hoffman AS, Schoen FJ, Lemons J (2020) Introduction-biomaterials science: An evolving multidisciplinary endeavor. Biomaterial’s Science: An Introduction to Materials in Medicine pp. 3-19.
- Hsiue GH, Hsu SH, Yang CC, Lee SH, Yang IK (2002) Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials 23(2): 457-462.
- Groth TP, Falck, Miethke RR (1995) Cytotoxicity of biomaterials-basic mechanisms and in vitro test methods: A Alternatives to Laboratory Animals 23(6): 790-799.
- Sukumaran A, Vishnupriya K, Sweety, Biba Vikas, Betsy Joseph (2023) Cytotoxicity and cell viability assessment of Cytotoxicity-Understanding Cellular Damage and Response.
- Borenfreund E, Borrero O (1984) In vitro cytotoxicity assays. Potential alternatives to the Draize ocular allergy test. Cell Biology and Toxicology 1(1): 55-65.
- Griffith M, Jackson WB, Lagali N, Merrett K, Li F, et al. (2009) Artificial corneas: A regenerative medicine approach. Eye 23(10): 1985-1989.
- Ratner BD (2004) Biomaterial’s science: An introduction to materials in medicine. San Diego, California, pp. 162-164.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65(1-2): 55-63.
- Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnology Annual Review 11: 127-152.
- Ciapetti G, Pratelli LCE, Pizzoferrato A (1993) In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 14(5): 359-364.
- Viera LMdAL, Silva RS, Silva CCD, Presgrave OAF, Boas MHSV, et al. (2022) Comparison of the different protocols of the Hen's Egg Test- Chorioallantoic Membrane (HET-CAM) by evaluating the eye irritation potential of surfactants. Toxicology in Vitro 78: 105255.
- Grabot, MB, Bernardin G, Chaumond S, Pinon JF (1995) Alternative methods: Hen's egg chorioallantoic membrane and in vitro cytotoxicity-a complementary approach. International Journal of Cosmetic Science 17(5): 207-215.
- Harsolekar M, Ansari M, Supe S, Singh K (2023) Formulation development and evaluation of therapeutic contact lens loaded with ganciclovir. International Ophthalmology 43(7): 2225-2236.
- Ribeiro A, Veigaet F, Santos D, Labandeira JJL, Concheiroal A, et al. (2011) Receptor-based biomimetic NVP/DMA contact lenses for loading/eluting carbonic anhydrase inhibitors. Journal of Membrane Science 383(1-2): 60-69.
- Pôbiš P, Kubalcová J, Milasová T, Kandárová H (2024) Development of sensitive in vitro protocols for the biocompatibility testing of medical devices and pharmaceuticals intended for contact with the eyes: Acute irritation and phototoxicity assessment. Alternatives to Laboratory Animals 52(5): 261-275.
- Kolle SN, Sauer UG, Moreno MCR, Teubner W, Wohlleben W, et al. (2016) Eye irritation testing of nanomaterials using the EpiOcular™ eye irritation test and the bovine corneal opacity and permeability assay. Particle and Fibre Toxicology 13(1): 18.
- Pellevoisin C, Coleman KP, Hoffmann S (2022) ISO 10993-23 In vitro irritation testing for medical devices: Substantiating applicability to mild irritants and non- Toxicology in Vitro 82: 105371.
- Lu R (2024) Advances in ophthalmic organ-on-a-chip models: Bridging translational gaps in disease modeling and drug scree International Journal of Translational Medicine 4(4): 710-725.
- Gensheimer T, Veerman D, Oosten EMV, Segerink L, Garanto A, et al. (2025) Retina-on-chip: Engineering functional in vitro models of the human retina using organ-on-chip technology. Lab on a Chip 25(5): 996-1014.
- Peng Z, Zhou L, Wong JKW, Chan YK (2020) Eye-On-a-Chip (EOC) models and their role in the future of ophthalmic drug discovery. Expert Review of Ophthalmology 15(5): 259-261.
- Fan L, Yang H, Yang J, Peng M, Hu J, et al. (2016) Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydrate Polymers 146: 427-
- Maulvi FA, Lakdawala DH, Shaikh AA, Desai AR, Choksi HH, et al. (2016) In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. Journal of Controlled Release 226: 47-56.
- Baino F, Perero S, Ferraris S, Miola M, Balagna C, et al. (2014) Biomaterials for orbital implants and ocular prostheses: Overview and future prospects. Acta Biomaterialia 10(3): 1064-1087.
- Piñero DP, Alcón N (2014) In vivo characterization of corneal biomechanics. Journal of Cataract & Refractive Surgery 40(6): 870-887.
- Mieler KJJ, Rudeen KM, Liu W, Mieler WF (2020) Advances in ocular drug delivery systems. Eye 34(8): 1371-1379.
- Lospa C, Rusu D (2002) Biomaterials in Ophthalmology: Intraocular lenses 1: 35-68.
- Kim SH, Jo SH, Kim BK, Park SH (2023) Tissue engineered mini-cornea model for eye irritation test. Tissue Engineering and Regenerative Medicine 20(2): 213-223.
© 2025 Andreza Maria Ribeiro. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






