- Submissions

Full Text
Innovation in Tissue Engineering & Regenerative Medicine
A Review of the Physiology, Market Analysis, and Treatments for Lipodystrophy
Ravindran Rushil and Tawil Bill*
Department of Bioengineering, University of California, USA Introduction
*Corresponding author:Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA USA
Submission: November 11, 2024;Published: November 25, 2024

Volume2 Issue1November 25, 2024
Abstract
Lipodystrophy results in the disorganization of adipose tissue which can result in the adipose tissue being lost in a specific region or throughout the body which can be due to multiple factors of genetics or medication. The market for Lipodystrophy itself looks promising and growth does seem apparent in order to find a cure, although it is classified as a rare disease. With many studies and publications suggesting potential that tissue engineering therapies may hold in being used to treat tissue engineering there is great promise. However, many past clinical trials and funding have provided inconclusive results for tissue engineering products and therapies in order to treat Lipodystrophy which indicates although there is promise for tissue engineering products. There is room for the field to develop and for more research to be conducted. The use of a product using a conventional tissue engineering approach of a scaffold, cells and growth factors with a type 1 collagen hydrogel with autologous-derived stem cells and VEGF may potentially prove to be useful in treating Lipodystrophy through the growth of adipose tissue. This review will look at the diseased and healthy tissue of Lipodystrophy, the market size and trends associated, the products available to treat this disease both existing and those currently in clinical trials as well as those in past clinical trials.
Keywords:Generalized; Acquired; Lipodystrophy; Adipose tissue; Biomaterials; Tissue engineering; Regenerative medicine
Abbreviations:BAT: Brown Adipose Tissue; CAGR: Compound Annual Growth Rate; CO2: Carbon Dioxide; FDA: Food and Drug Administration; FFA: Free Fatty Acids; HIV: Human Immunodeficiency Virus; IGF-1: Insulin-like Growth Factor 1; LEPR: Leptin Receptor; NIDDK: National Institute of Diabetes and Digestive and Kidney Diseases; PBS: Phosphate Buffered Saline; PLG: Poly(lactide-co-glycolide); NaCl: Sodium Chloride; VEGF: Vascular Endothelial Growth Factor; WAT: White Adipose Tissue
Introduction
Lipodystrophies are commonly associated with a loss of adipose tissue characterized by a loss of fat from various locations across the body [1]. Lipodystrophies are presented in multiple different types, that can be obtained from both acquired and genetic means, they can vary from the lack of partial versus generalized lack of adipose tissue along with the location as to where this tissue is lost [2]. Lipodystrophy can additionally be the result of antiretroviral therapy used to treat HIV patients that may develop Lipodystrophy as a result along with drugs such as insulin inducing lipodystrophy [3,4]. This loss of adipose tissue may pose a risk in resulting in the propagation of physiological symptoms failure along with endorgan damage which may pose a risk in having increased mortality in patients and increased pain due to tissue loss [5]. The disease itself appears to be a rare but deadly disease that can affect multiple patients that has no definite cure to restore the tissue that is once lost [4]. However, with advancements in research into orphan drugs and research initiatives for drugs the market is expected to grow and develop in the coming future along with the development of new treatments [4]. Treatments such as artificial recombinant leptin, metreleptin, have been present and upcoming in order to treat patients in order to restore their metabolic levels from the lack of adipose tissue to normal with the limited use of medication [3,4,6]. Tissue engineering solutions may prove to be able to improve adipose tissue regeneration using regenerative approaches in order to improve adipose tissue growth and deficiency [7]. This review will aid to view the tissue engineering aspect of lipodystrophy but also look at the market size and growth of the disease and discuss the treatments for it.
Healthy tissue
Adipose tissue itself is primarily composed of adipocytes that make up the largest cell volume in the tissue being the defining cell type of Adipose Tissue [8]. Adipose Tissue itself is divided into brown and white adipocytes that have physiological differences that give rise to different functions located in various locations throughout the body (Figure 1D) [8]. Brown Adipose Tissue (BAT) has been found to regulate the temperature of the body and lead to its maintenance [8]. BAT uses thermogenic regulation by dissipating chemical energy as heat through UCP1 as well as burning lipids [9]. UCP1 functions by relieving the proton gradient in the inner mitochondrial membrane thus releasing heat from the uncoupling mechanism of ATP synthesis [10,11]. Beige adipocytes are also another subtype of adipose tissue that exists, which are similar to function and structure brown adipocytes that are found in White Adipose Tissue (WAT) that also have a thermogenic ability to release heat throughout the body [11]. However, Beige adipocytes require external stimulation from climate, exercise, or adrenergic signals in order to stimulate their thermogenic capability [11].
White adipose tissue is the sole largest contributor of adipose tissue in mammals and is responsible for functions of insulin sensitivity, storing energy, and endocrine signaling and communication (Figure 1C) [8]. Adipocytes in WAT typically phenotypically express a single, large lipid droplet that is seen to occupy most of the cell volume with less mitochondria [10]. The purpose of this phenotypic expression is to store and release energy through lipolysis or lipogenesis which results in the contributing factor for adipose tissue expansion [10]. Adipose tissue uses adipocytes to store body fat as triacylglycerols under energy surplus and release fatty acids and hormones to the rest of the body during high or low energy needs [8]. Adipocytes are also able to regulate blood glucose levels through their sensitivity to insulin (Figure 1C) to stimulate the uptake of glucose and help regulate its levels [8].
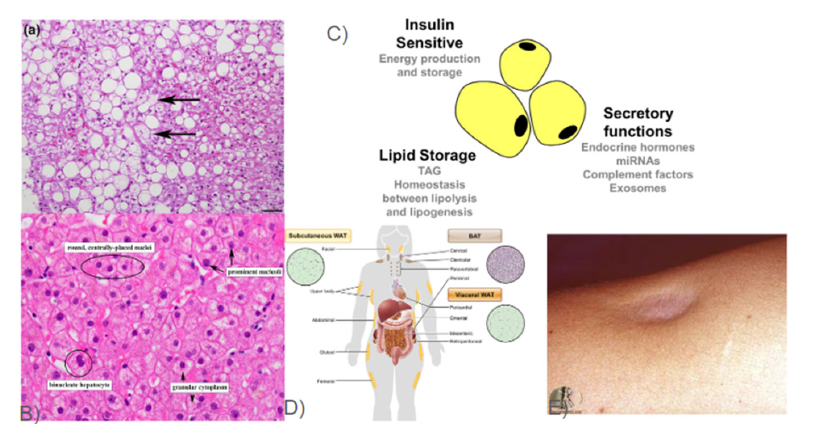
Figure 1:Physiological functions and locations of adipocytes in the human body and the healthy versus diseased
tissues [8,16,17,32].
This image shows: 1A) Liver histology micrograph of patient affected with lipodystrophy [17] 1B) Healthy liver
histology slide showing hepatocytes [43] 1C) List of some of the three main Physiological Functions that Adipocytes
serve [8] Figure 1D) Model of the locations of white and brown adipose tissue deposits shown in humans [44] 1E)
Dermatologic visualization of localized lipodystrophy [16].
Other than just adipocytes, adipose tissue is home to preadipocytes, macrophages, endothelial cells, leukocytes, and fibroblasts that are able to be stored for the purpose of controlling metabolism [9]. Through adipose tissue’s role in energy storage, it is able to use the stored triglycerides from surplus energy to be broken down to glucose and fatty acids for times when the body has a decreased food intake that can be transported throughout the body to organs in order to help promote functions and decrease energy loss [9]. Adipose tissue’s other niche in its integration with the endocrine system further enables its interactions with organ systems to use bioactive factors such as adipokines-cytokines be used through binding to extracellular membrane receptors in order to result in conformational changes which trigger signaling pathways modulating metabolic activity for homeostasis [9].
Within the tissue itself, the signaling and use of adipokines play a role in the regulation of cross body metabolism and signaling [12]. Adipokines play this role by promoting insulin sensitivity, through adiponectin, insulin resistance, and inflammation through the secretion of Interleukins 6,1β, 8,18 as well as TNF-ɑ.46 Leptin, as an adipokine, is produced by white adipose tissue and acts as a marker in order to reflect the amount of energy that the body stores, reducing gluconeogenesis in both adipose tissue and the liver to decrease the levels of glucose released [12,13]. Leptin is also able to protect peripheral tissues from lipotoxicity by stimulating fatty oxidation and is also able to reduce lipid accumulation in the liver and in muscles [12]. The concentration of leptin helps the body to respond when leptin is low in order to help the body deal with starvation and trigger responses, When leptin is high the body increases satiety to reduce food intake and increase the energy spent to decrease the high levels of energy present [13].
Diseased tissue
Lipodystrophy is different in its pathophysiology from healthy adipose tissue through the presence of adipose tissue [14]. From the physical presentation of lipodystrophy, it is a rare disease that is clinically diagnosed by its symptoms through examination or via imaging, such as ultrasound, which often contributes to delays and errors in diagnosis [5]. As adipose tissue’s absence allows for decreased metabolic activity and fat storage due to the lack of fat cells which could be either through the mechanism of not being differentiated into adipocytes or triggered cell death, through apoptosis [14]. Due to the absence of adipose tissue in its role for metabolic activity, as an endocrine organ, fat cells are unable to store lipids since they are absent so lipids are instead stored ectopically in non-fat cells resulting in an imbalance in adipose tissue and fatty acids [14]. Lipodystrophy can be caused by infections, medications, autoimmune disorders through acquired means but also be the result of a genetic recessive gene to be considered congenital [5]. Lipodystrophy results in diseased tissue that is categorized by partial or generalized Lipodystrophy dependent on the degree of the loss of adipose tissue [5]. With the role that adipose tissue serves, lipodystrophy results in a loss of this tissue which results in the loss of the metabolic role that the tissue serves leading to increased insulin resistance, ectopic steatosis, severe dyslipidemia and organ dysfunction [5]. This lack of tissue can result in diseased tissue that lacks a surface of fat in partial Lipodystrophy, this can result in a crater or deformed regions of skin and tissue (Figure 1E) [15,16]. The distribution of the adipose tissue loss also contributes to the physiological effects of lipodystrophy (Figure 1D) [12]. Patients with lipodystrophy commonly show metabolic complications which show the importance of adipose tissue in its function in the endocrine system in secretion of hormones (Figure 1C) and signaling [12]. It is important to note that lipodystrophy does not have consequences of its own but the dire results associated stem from metabolic abnormalities that stem from the loss of adipose tissue [5]. Where the consequences are dependent on the type of lipodystrophy be it partial or generalized, along with the individual’s age and gender [5]. Lipodystrophy not only results in a loss of adipose tissue inhibiting metabolic function and decreased organ function without the key endocrine component but also results in low levels of Leptin which has been associated with this loss of function [5,12]. A lack of adipose tissue can result in systemic effects to other tissues and the body, which can be due to inflammation and swelling of tissue throughout which can lead to fibrosis [5,17]. The lack of adipose tissue can reach the liver and result in liver failure due to steatohepatitis with ballooning, inflammation and steatosis, the accumulation of fat of diseased tissue (Figure 1A) compared to the healthy liver tissue with intact hepatocytes, no loss or swelling of tissue visible (Figure 1B) [17].
Market Size, Products, and Companies
Market size
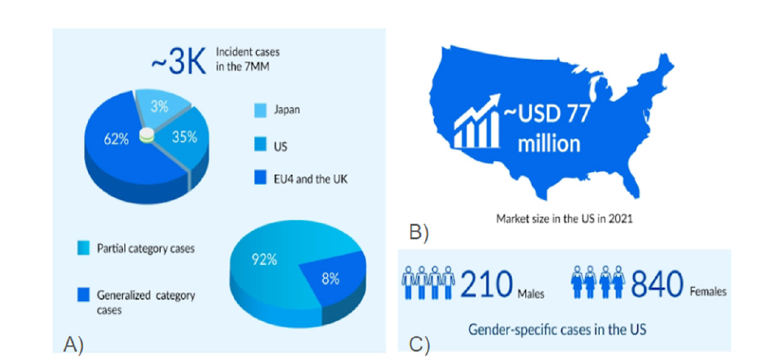
The global prevalence of Lipodystrophy has been stated to be less than 1 case in 1,00,000 people making it quite a rare disease as per Lipodystrophy United [4]. In the U.S. alone there has been estimated to be less than 200,000 cases for patients [4]. According to an article published in the Journal of Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, the prevalence of lipodystrophy was indicated to be 2.63 cases/million people in Europe [4]. Although Lipodystrophy does not directly result in death, it is associated with reducing the average lifespan by 30 or more years with the most common cause of death being from liver disease and failure as well as infection in congenital lipodystrophy [18]. Lipodystrophy in the United Kingdom, and Germany, Spain, Italy, France constituted 62% of the cases, 1,850 cases of lipodystrophy studied in the seven major markets of the United States, Germany, Spain, Italy, France, the U.K., and Japan [19,20]. While in the United States the number of cases in 2021, 1,050 cases constituted 35% (Figure 2A) in the seven major markets [19,20].
In the United States, as of 2021 there were 210 males and 840 females (Figure 2C) associated with having lipodystrophy [20]. Within the seven major markets there were 2,300 females associated with lipodystrophy whereas for males there were 700 cases in 2021 [19]. The market itself for Lipodystrophy has been shown to be worth at least 120 million dollars worth over the course of only four of the seven major markets of Lipodystrophy, with the United States having the largest market making up 77 million dollars USD (Figure 2B) [19]. The age for Lipodystrophy has been shown to be diagnosed varying upon if the disease is genetic or acquired, if acquired then the median age for Lipodystrophy is 25 years of age [7]. For genetic Lipodystrophy, the loss of adipose tissue may start at birth and could be diagnosed early due to the autosomal disorder and may be diagnosed as the teenage years of life [5].
Figure 2:Statistics and market report of the market size and cases in patients with lipodystrophy [20]. This image shows: A) Cases of lipodystrophy by percentage within the US, Japan, EU4, consisting of Germany, Spain, Italy, and France along with percent of patients associated with partial versus generalized lipodystrophy [20] B) Market size of the lipodystrophy market in the United States in 2021 [20] C) Cases of lipodystrophy by gender within the United States [20].
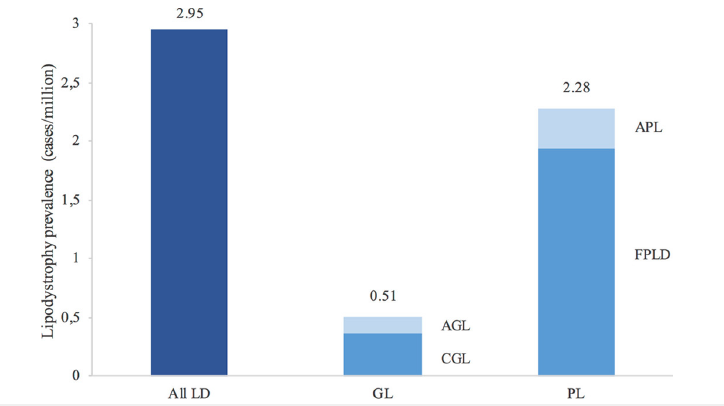
Depending upon the type of Lipodystrophy, the number of patients that might have the disease or be diagnosed for it will differ (Figure 3) with Generalized Lipodystrophy being much less prevalent in the country of Spain compared to Partial Lipodystrophy which is Familial partial Lipodystrophy [21]. The 2.95 cases per million (Figure 3) are shown within Spain that fall within the expected ranges of 1.3–4.7 cases per million for generalized lipodystrophy of 0.2–1.0 cases/million whereas for partial lipodystrophy the number of cases fall in 1.7–2.8 cases per million [21].
Figure 3:Prevalence of lipodystrophy in Spain [21]. The image shows a bar graph of the estimated prevalence of cases of lipodystrophies in Spain from 2020 according to the Spanish National Statistics Institute [21].
Market trends
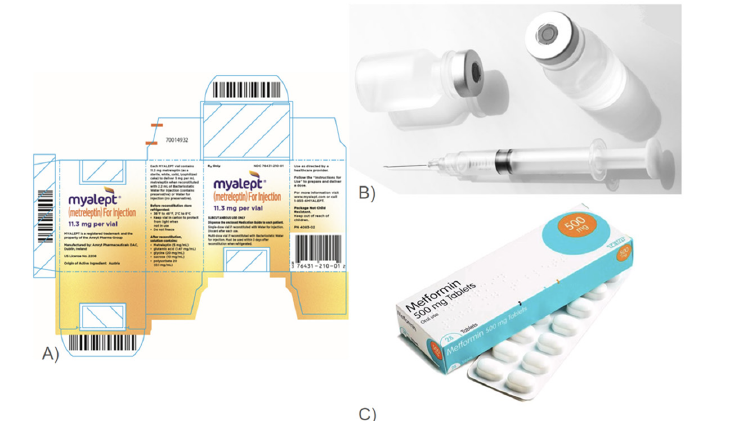
With the number of patients for Lipodystrophy being very small for both partial and generalized Lipodystrophy the FDA classifies Lipodystrophy as a rare disorder in order to be applied under the Orphan Drug Act, specifically for the authorization of metreleptin [4,22]. With advancements in a high return on orphan drugs such as metreleptin, research and development initiatives are predicted to forward and develop the market as there is no cure [4]. With the disease’s increasing prevalence over the coming years along with its awareness, the market is expected to surge due to the expected increase in the number of cases [19]. With the launch of new pipeline products for Lipodystrophy and products in clinical trials the market dynamics is expected to be revolutionized [19]. The epidemiology based on gender indicates that females have a higher prevalence of Lipodystrophy compared to males [19]. Over the course of 2021 to 2028 the Compound Annual Growth Rate (CAGR) is predicted to be 5.80% [1]. The key companies at the center developing new drugs and approaches to combat Lipodystrophy consist of: Ionis Pharmaceuticals, Regeneron Pharmaceuticals, Boehringer Ingelheim, Gilead Sciences, Regeneron Pharmaceuticals, Amryt Pharma, Akcea Therapeutics, Theratechnologies, Glaxo Wellcome, Merck Sharp & Dohme LLC, Pfizer, Regeneron Pharmaceuticals, Rhythm Pharmaceuticals, Inc., Eli Lilly and Company, Sanofi, and others [19,20] (Figure 4).
Figure 4:Existing product images of myalept and metformin [45-47]. A) Packaging and prescription for the common name brand metreleptin, myalept [45] B) Vial and injection used for metreleptin in patients [46] C) Packaging and images of the Metformin medication [17].
Existing products
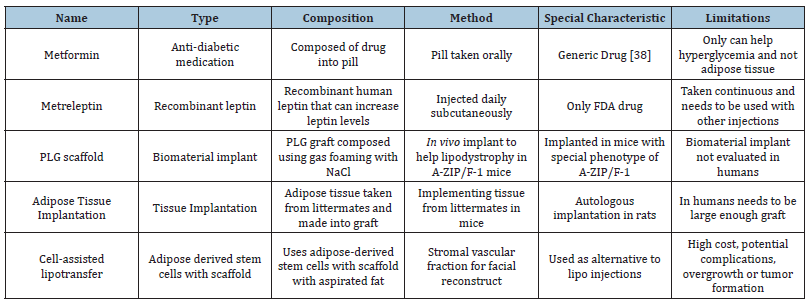
The only FDA approved medication to treat Lipodystrophy is metreleptin, injected daily, which uses recombinant methionyl human leptin and is able to increase low levels of leptin resulting in improvements in hypertriglyceridemia, glycemic control, and liver volume [6,23]. Lifestyle intervention along with physical exercise is another initial treatment in order to help with lipodystrophy by having a balanced macronutrient diet with 50-60% carbohydrates, 20-30% fat and 20% protein [24]. In order to combat the hyperglycemia that may result from lipodystrophy as well as insulin resistance metformin has been shown to be a good drug that can help improve symptoms associated with diabetes [24]. Cosmetic surgery and intervention can also be used in order to provide more adipose tissue through using plastic surgery with autologous tissue transplantation, reconstruction using implants with areas with low adipose tissue [5].
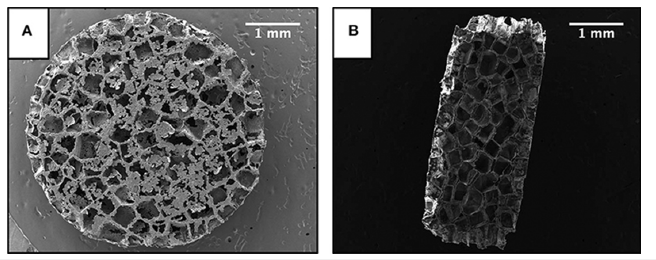
With the loss of adipose tissue in Lipodystrophy, the accumulation of lipid is stored in non-adipose tissue is known as ectopic lipid [25]. In order to treat this problem of ectopic lipid damaging organs and inhibiting their function, scaffolds composed of Poly(lactide-co-glycolide) that were implanted in vivo in the epididymal fat of mice [25]. The scaffold (Figure 5A,5B) was created using gas foaming using CO2 at room temperature using PLG and 250-500μm NaCl particles [25]. The mechanism of the scaffold was proposed to be the interaction between the biomaterial implant in vivo will allow for integration between the adipose tissue and the scaffold [25]. The mice with the scaffold were fed a high fat diet in order to induce ectopic lipids which resulted in a decrease in triglycerides by 53% in the gastrocnemius and a decrease of 25% in the liver [25].
Figure 5:Schematic example of a typical bioink preparation process [10].
The image shows an example of a typical bioink preparation process for GelGMA. Gelatin was dissolved in distilled
water and allowed to react with GMA overnight. Subsequently, to eliminate salts, the bioink solutions underwent
dialysis against distilled water, followed by freeze drying.
Images of Scaffold taken using Scanning Electron Microscope [25]. The image shows in A) The top view of the
scaffold from SEM [25] and in B) A cross-Section of scaffold from SEM37.
In order to reverse lipoatrophic diabetes, a loss of adipose tissue encompassed by lipodystrophy, implantation of wild type adipose tissue was done in A-ZIP/F-1 mice, which have a severe form of lipoatrophic diabetes [26,27]. The adipose tissue was taken from littermates of the mice, where the tissue was placed into sterile PBS and proceeded to be cut into 100-150mg pieces, where they were implanted subcutaneously into the mice through small incisions in the shaved skin of the back (Figure 6A) [27]. The implantation of adipose tissue in these mice was able to reverse the hyperglycemia, dramatically lower insulin levels, and improve muscle insulin sensitivity associated with lipoatrophy [27]. The levels of triglycerides and FFA were of modest and not significant improvement in mice that have the adipose tissue implanted [27]. With the use of adipose tissue implantation, the diabetes can be reversed using this surgical reconstitution in and can be made applicable to patients with humans, if the graft is large enough then patients with genetic lipoatrophy should benefit from adipose tissue transplantation [27].
Figure 6:Use of tissue implantation and cells as a solution in cases. This image shows A) Overview of the procedure of cell-assisted lipotransfer [28] B) Three weeks after Implantation of adipose tissue into rats [46].
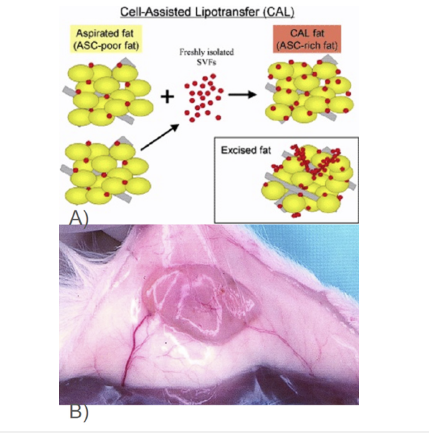
In order to treat facial lipoatrophy in human patients, cell assisted lipotransfer has found possible use in the efficacy and safety of the procedure in order to restore adipose tissue in the face (Figure 1B) for facial recontouring [28]. The mechanism of cellassisted lipotransfer functions is to use adipose-derived stem cells in order to construct adipose grafts for reconstruction of tissue [28]. Cell-assisted lipotransfer uses a stromal vascular fraction with aspirated fat which is separated into two fractions (Figure 6B), one which is removed of its fat and attached to the other fraction of the aspirated fat which function as a scaffold [28] (Table 1).
Table 1:Treatments and existing products to combat lipodystrophy and the complications that may arise associated with it [5,6,23-25,27,28,48].
Ongoing Studies and Clinical Trials
One study aimed to show that the implantation of the potential use of autologous adipose-derived stem and progenitor cells from patients with Lipodystrophy in order to regenerate adipose tissue to which they showed sufficient differentiation potential by assessing the lipid droplet formation from the cells along with physiological and biological activity to aid in the reconstitution of adipose tissue [29]. There have been multiple clinical trials that have been conducted using tissue engineering therapies in order to treat lipodystrophy, however their results are not shown or the studies diverge from the original purpose to use tissue engineering in the aid of lipodystrophy [29-31]. The use of adipose stem cells has been reported to be integrated in clinical trials that have been shown to be completed in order to help with soft-tissue augmentation [32]. In Brazil, a clinical trial was conducted in order to aim to determine the safety of the autologous transplantation of adipose-derived stem cells in order to help combat lipodystrophy [29]. Another clinical trial sponsored by King Edward Medical University in 2015 attempted to use mesenchymal stem cells in order to be used for fat grafting through the use of adipose tissue-derived mesenchymal stem cells [30]. Another clinical trial conducted across the United States evaluated the effects of using Lamivudine/Zidovudine, Abacavir sulfate, Nelfinavir mesylate in order to evaluate the changes in body fat for HIV patients [33]. A clinical trial conducted by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) evaluated the short term effects of leptin withdrawal or initiation with metreleptin in Lipodystrophy independent of energy consumption [34]. A clinical trial was conducted by EMD Serono across the United States in order to evaluate the effectiveness of Serostim in order to help treat the abnormal fat accumulation and distribution associated with HIV-associated Adipose Redistribution Syndrome which was found from the results to reduce adipose tissue with Serostim more than placebo [35].
With ongoing clinical trials, there is a study that has finished and being analyzed conducted by the National Institute of Diabetes and Digestive and Kidney Disease using REG4661, a Leptin Receptor Agonist Antibody, in order to increase the potential amount of leptin in patients with generalized lipodystrophy that will help to reduce blood sugar and triglyceride levels [36,37]. REG4661 is able to activate the LEPR in the presence or absence of leptin which has been associated to result in mouse models that alleviate symptoms of hyperglycemia, insulin resistance, and hepatic steatosis [38]. Additionally this use of REG4661 is being testing patients with familial partial lipodystrophy conducted also by the National Institute of Diabetes and Digestive and Kidney Disease and same Principal Investigator to evaluate if REG4661 is an effective treatment in familial partial lipodystrophy [36,39]. A clinical trial is currently being started with participants being currently recruited/ evaluated for phase II in order to evaluate if the use of Pegvisomant, growth hormone inhibition, would lead to patients recovering from severe insulin resistance with patients that have partial dystrophy or a known variant in the insulin receptor gene [37,40]. Pegvisomant functions as a genetically engineered growth hormone receptor antagonist in order to block the growth hormone concentrations resulting in lower concentrations of IGF-1 [41].
Conclusion and Future Considerations
The market size of Lipodystrophy appears to be at least 120 million globally with 77 million (Figure 2B) estimated in the United States, however the prevalence of cases per population classifies the disease as rare and under the orphan drug act with more females being affected than males [19,4]. The emphasis on the benefits of the orphan drug act and increased funding in research along with developments in techniques and approaches are all expected to drive this market further for Lipodystrophy for investment and companies to develop new solutions [4,42].
With the effects of Lipodystrophy affecting patients leading to a loss of adipose tissue being a metabolic disorder that can result in a widespread disease, the prevalence of drugs continues to increase for this rare disease with metreleptin being the only FDA approved drug [2,4,5]. With current clinical trials underway for REG4661 and Pegviosmant the market is expected to grow further with the approval of continued drugs to expand even further within the next decade with a CAGR of 5.80% [1,36-39]. With the development of tissue engineering and stem cell therapies, applications have been made using autologous cells and transplantation as well as cell assisted lipotransfer for facial dystrophy (Figure 6A) [5,7,24- 29]. Past clinical trials and experimentation of available methods suggest the possibility for the effectiveness of tissue engineering approaches in order to treat lipodystrophy using adipose tissue engineering techniques in humans [4,7,27-32]. One prospective avenue for the field based on clinical trials and developments into treatment could possibly to use scaffolds, cells, and growth factors in order to build adipose tissue as a potential treatment. However more experimentation needs to be done with credible results in order to develop new potential therapies and advance the market [4,7,27-30,32,43-48].
Acknowledgements
Rushil Ravindran expresses appreciation to Professor Bill Tawil for overseeing the framework of this review, and for the insightful lectures and advice concerning biomaterials and tissue engineering, which contributed to the development of this paper (Appendix 1 & 2).
Appendix 1. Companies:
1a. Ionis Pharmaceuticals: https://www.ionis.com/
1b. Regeneron Pharmaceuticals: https://www.regeneron.com/
1c. Boehringer Ingelheim: www.boehringer-ingelheim.com/
1d. Gilead Sciences: www.gilead.com/
1e. Amryt Pharma: www.amrytpharma.com/
1f. Akcea Therapeutics: www.akceatx.com/
1g. Theratechnologies: www.theratech.com/
1h. Merck Sharp & Dohme (MSD): www.msd.com/
1i. Pfizer: www.pfizer.com/
1j. Rhythm Pharmaceuticals: www.rhythmtx.com/
1k. Eli Lilly and Company: www.lilly.com/
1l. Sanofi: www.sanofi.com/
2. Products
2a. Metformin®:
2b. Metreleptin®: https://www.myalept.com/
References
- (2024) Acquired Lipodystrophy Treatment Market – Global Industry Trends and Forecast to 2028. Data Bridge Market Research. Market Research Business Consulting and Strategy Planning Firm, Data Bridge Market Research Private Ltd,.
- UT Southwestern Medical Center.
- Quinn K, Chauhan S, Purcell SM (2023) Lipodystrophies. StatPearls.
- Lipodystrophy treatment market: Increase in the number of rare diseases related to genetic mutations to drive the market. BioSpace.
- Akinci B, Gular MC, Oral EA (2024) Lipodystrophy syndromes: Presentation and treatment.
- Brown RJ, Oral EA, Cochran E, Araújo-Vilar D, Savage DB (2018) Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 60(3): 479-489.
- Suzuki K, Akita S, Yoshimoto H, Ohtsuru A, Hirano A, et al. (2024) Biological features implies potential use of autologous adipose-derived stem/progenitor cells in wound repair and regenerations for the patients with lipodystrophy. Int J Mol Sci 20(21): 5505.
- Richard AJ, White U, Elks CM, Stephens JM (2024) Adipose tissue: Physiology to metabolic dysfunction. Endotext - NCBI Bookshelf. National Center for Biotechnology Information.
- Luo L, Liu M (2016) Adipose tissue in control of metabolism. J Endocrinol 231(3): R77- R99.
- Sakers A, Siqueira MK, Seale P, Villanueva CJ (2022) Adipose-tissue plasticity in health and disease. Cell 185(3): 419-446.
- Yang Liu, Qian S, Yan Tang, Tang Q (2024) The secretory function of adipose tissues in metabolic regulation. Life Metabolism 3(2): loae003.
- Fiorenza CG, Chou SH, Mantzoros CS (2024) Lipodystrophy: Pathophysiology and advances in treatment. Nature 7: 137-150.
- Dornbush S, Aeddula NR (2023) Physiology, leptin. StatPearls.
- Knebel B, Müller-Wieland D, Kotzka J (2020) Lipodystrophies—disorders of the fatty tissue. International Journal of Molecular Sciences 21(22): 8778.
- (2024) Lipodystrophy: What it is, symptoms, types & treatment. Cleveland Clinic.
- Schwartz RA, Isabelle Thomas (2023) Dermatologic manifestations of localized lipodystrophy. Medscape.
- Figure 3. Histology of the pancreas, liver and kidney.
- Lima JG, Nobrega LHC, Lima NN, Santos MCFD, Silva PHD (2024) Causes of death in patients with berardinelli-Seip congenital generalized lipodystrophy. PLoS One 13(6): e0199052.
- GetNews (2024) lipodystrophy market is predicted to exhibit remarkable growth during the forecast period (2023-2032), Analyzes DelveInsight | Boehringer Ingelheim, Gilead Sciences, Aegerion Pharma, Regeneron Pharma. Barchart.
- (2024) Lipodystrophy - Market insight, epidemiology and market forecast - 2034. Delveinsight, pp. 1-107.
- Fernández-Pombo A, Sánchez-Iglesias S, Castro-Pais AI, Ginzo-Villamayor MJ, Cobelo-Gómez S (2024) Natural history and comorbidities of generalised and partial lipodystrophy syndromes in Spain. Front Endocrinol (Lausanne) 14:1250203.
- US Food and Administration, Search orphan drug designations and approvals.
- Musso C, Major ML, Andres E, Simha V (2024) Metreleptin treatment in three patients with generalized lipodystrophy. Clin Med Insights Case Rep 9: 123-127.
- Mainieri F, Tagi VM, Chiarelli F (2024) Treatment options for lipodystrophy in children. Front Endocrinol (Lausanne) 13: 879979.
- Hendley MA, Isely C, Murphy KP, Hall HE, Annamalai P, et al. (2024) Scaffold implant into the epididymal adipose tissue protects mice from high fat diet induced ectopic lipid accumulation and hyperinsulinemia. Front Bioeng Biotechnol 8: 562.
- Oral EA, Raja-Khan N (2024) Generalized lipodystrophy: Practice essentials, pathophysiology, lipoatrophic diabetes. Medscape.
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, et al. (2024) Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. The Journal of Clinical Investigation 105(3): 271-278.
- Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, et al. (2008) Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 34(9): 1178-1185.
- Clinical Trials (2022) Autologous Adipose-Derived Stem Cell Transplantation in Patients With Lipodystrophy (AADSCTPL).
- Clinical Trials (2015) Role of mesenchymal stem cells in fat grafting. Muhammad Mustehsan Bashir, King Edward Medical University (Responsible Party).
- Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ (2012) Human adipose stem cells: Current clinical applications. Plastic and Reconstructive Surgery, pp. 1277-1290.
- Mughal M, Sindali K, Man J, Roblin P (2024) Fat chance: A review of adipose tissue engineering and its role in plastic and reconstructive surgery. Ann R Coll Surg Engl 103(4): 245-249.
- Clinical Trails (2005) A comparison of three anti-HIV Drug combinations in HIV-infected patients. NIH AIDS Clinical Trials Information Service.
- Clinical Trials (2019) Short-term effects of leptin in people with lipodystrophy. National Institutes of Health Clinical Center (CC) (National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)) (Responsible Party).
- Clinical Trails (2018) Treatment of abnormal adipose tissue accumulation in Human Immunodeficiency Virus (HIV) Patients. EMD Serono (Responsible Party).
- A randomized, double-blinded, placebo-controlled study of REGN4661, A leptin receptor agonist antibody, in patients with generalized lipodystrophy. NIH Clinical Center.
- NIH Clinical Center: Search the studies.
- Altarejos JY, Pangilinan J, Podgrabinska S, Akinci B, Foss-Freitas M, et al. (2024) Preclinical, randomized phase 1, and compassionate use evaluation of REGN4461, a leptin receptor agonist antibody for leptin deficiency. Sci Transl Med 15(723): eadd4897.
- (2024) NIH Clinical Center: Search the studies.
- Phase II study of growth hormone inhibition using pegvisomant in severe insulin resistance. NIH Clinical Center: Search the Studies.
- Lee AP, Mulligan K, Schambelan M, Murphy EJ, Weiss EJ (2024) Growth hormone receptor antagonism with pegvisomant in insulin resistant non-diabetic men: A phase II pilot study. F1000Res 6: 614.
- (2022) North America can be expected to register rapid revenue growth of lipodystrophy market over the forecast period 2022-2030. BioSpace.
- Phoenix Bell (2020) Normal liver histology 101. AASLD.
- Lim K, Haider A, Adams C, Sleigh A, David B (2024) Lipodistrophy: A paradigm for understanding the consequences of “overloading” adipose tissue. American Physiological Society 101(3): 907-993.
- DailyMed (2014) These highlights do not include all the information needed to use MYALEPT safely and effectively. See full prescribing information for MYALEPT. MYALEPT® (metreleptin) for injection, for subcutaneous use initial U.S. Approval.
- Haymarket Media (2024) Myalept approved for leptin deficiency. Clinical Advisor.
- Alex G (2024) Has your doctor prescribed you metformin? Let us tell you what you need to know about taking metformin. My Health Explained.
- (2024) Metformin: Side effects, dosage, uses, and more. Medical and Health Information.
© 2024 Tawil Bill. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






