- Submissions

Full Text
Intervention in Obesity & Diabetes
The Key Role of Finerenone in the Management of Diabetic Kidney Disease
Divisón-Garrote JA1*, Turégano-Yedro M2 and Pallarés-Carratalá V3
1Professor Medical Degree, Department of Medicine Universidad Católica Murcia (UCAM), Spain
2Primary Care, Casar de Cáceres Health Centre, Spain
3Department of Medicine, Universitat Jaume I, Spain
*Corresponding author:Divisón-Garrote JA, Professor Medical Degree, Department of Medicine Universidad Católica Murcia (UCAM), Primary Care, Casas Ibáñez Health Care Centre, Albacete, Murcia, Spain
Submission:November 03, 2025;Published: November 21, 2025

ISSN 2578-0263Volume7 Issue1
Opinion
Diabetic Kidney Disease (DKD) represents one of the most significant microangiopathic complications of Diabetes Mellitus (DM). It is characterized by progressive renal damage in individuals with Type 2 Diabetes (T2D), clinically defined by the persistent presence of albuminu-ria (or proteinuria) and/or a sustained reduction in Estimated Glomerular Filtration Rate (eGFR) for at least three months [1], in the absence of other primary causes of kidney disease [1]. Currently, DKD is one of the leading causes of End-Stage Kidney Disease (ESKD) and renal replacement therapy worldwide, which emphasizes the critical importance of early screening, prompt diagnosis and timely initiation of therapy to prevent these outcomes [1]. For several decades, numerous treatments have been employed to slow DKD progression, with special emphasis on Renin-Angiotensin-Aldosterone System (RAAS) blockade (ACE inhibitors and ARBs) and more recently, Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i). Classic RAAS blockers such as captopril, losartan or irbesartan reduced DKD progression; in the RENAAL trial, losartan decreased the risk of doubling serum creatinine by 25% and the incidence of ESKD by 28% [2]. SGLT2i have since demonstrated robust renoprotective and cardioprotective benefits beyond RAAS inhibition, significantly reducing mortality and progression to ESKD [3], thus becoming an indispensable cornerstone in DKD management. Both dapagliflozin and empagliflozin have shown a significant reduction in the risk of Chronic Kidney Disease (CKD) progression in patients with and without diabetes, thereby establishing themselves as key therapeutic agents regardless of glycemic status [4,5].
However, despite the widespread use of RAAS blockers and the transformative impact of SGLT2 inhibitors, a substantial proportion of patients continue to exhibit persistent albuminuria, progressive eGFR decline, and disproportionate Cardio Vascular (CV) risk. Against this backdrop, finerenone, a selective Nonsteroidal Mineralocorticoid Receptor Antagonist (ns-MRA), has emerged as a novel therapy with robust evidence for simultaneously attenuating renal disease progression and reducing cardiovascular events in DKD [6]. Unlike steroidal MRAs (spironolactone and eplerenone), finerenone offers greater receptor selectivity, favorable binding affinity, a shorter half-life without active metabolites and improved tissue distribution [7], translating into superior cardiorenal benefits and a lower risk of hyperkalemia. The pathophysiologic rationale for finerenone in DKD is grounded in mineralocorticoid receptor blockade across renal, cardiac and vascular tissues [8,9].
This blockade leads to anti-inflammatory and antifibrotic effects-partly by reducing proinflammatory cytokine production and thereby slowing glomerular, tubular and interstitial injury while reducing oxidative stress and proteinuria [8,9]. In FIDELIO-DKD [6], a double-blind randomized controlled trial including 5,734 patients with DKD receiving optimized RAAS blockade, finerenone reduced the primary composite outcome (progression to ESKD, sustained ≥40% decline in eGFR or renal death) by 18% compared with placebo. Subsequently, FIGARO-DKD [10], which enrolled a complementary cohort with a broader spectrum of CV and renal risk, confirmed the superiority of finerenone in reducing the primary CV composite endpoint (time to first CV death or nonfatal cardiovascular event such as myocardial infarction, stroke or hospitalization for heart failure) by 13%. The pooled FIDELITY analysis [11], integrating individual data from both trials (>13,000 participants), reinforced these consistent benefits across renal and cardiovascular outcomes, demonstrating a substantial reduction in overall cardiorenal burden.
These findings firmly establish finerenone as a dual cardiorenal protective agent, complementary to RAAS blockade and SGLT2 inhibition, within an integrated therapeutic framework for DKD. Consequently, major clinical practice guidelines have incorporated finerenone: the KDIGO 2025 guidelines recommend its addition in patients with T2D, CKD, and persistent albuminuria despite optimized RAAS blockade and SGLT2i therapy, provided potassium levels and eGFR are within acceptable limits [12]. Similarly, the American Diabetes Association (ADA) Standards of Care 2025 position finerenone as a first-line therapy to reduce both renal progression and cardiovascular events in patients with T2D, CKD and albuminuria [13].
Beyond its individual benefits, the synergistic effects of combining RAAS blockade, SGLT2 inhibitors and ns-MRA have been a subject of increasing interest.
The CONFIDENCE trial [14] evaluated the simultaneous initiation of finerenone and empagliflozin versus monotherapy in patients with DKD. At 180 days, the combination achieved a 52% reduction in Urinary Albumin-to-Creatinine Ratio (UACR), representing an additional 29% reduction vs finerenone alone and 32% vs. empagliflozin monotherapy, with a safety profile comparable across subgroups. Over one-third of participants achieved a ≥30% UACR reduction within the first 14 days of treatment [14]. These results support the implementation of a triple therapy approach (RAAS blockade+SGLT2i+finerenone), with appropriate monitoring of serum potassium and renal function.
Finally, the fourth cornerstone in DKD management comprises GLP-1 receptor agonists (GLP-1 RA)-particularly semaglutide. The FLOW trial [15], enrolling 3,533 patients with T2D and CKD (most on RAAS blockers and some on SGLT2i), demonstrated that weekly semaglutide 1mg reduced the risk of the primary composite endpoint (sustained ≥50% eGFR decline, progression to ESKD or renal or CV death) by 24%, CV mortality by 29% and all-cause mortality by 20%. Beyond glycemic control and weight loss, semaglutide thus delays DKD progression and reduces major cardiovascular events-particularly beneficial in patients with BMI ≥30kg/m². These findings reinforce the role of GLP-1 RA as complementary cardiorenal therapy alongside RAAS blockade, SGLT2 inhibitors and finerenone.
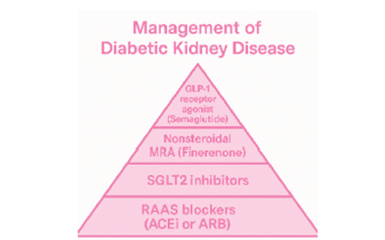
Consequently, contemporary management of DKD now embraces a four-pillar strategy-RAAS blockade, SGLT2 inhibition, finerenone and GLP-1 receptor agonists-which, when applied in combination, provides a greater reduction in cardiorenal risk than any single agent alone [16] (Figure 1). In conclusion, DKD remains one of the leading causes of ESKD, underscoring the priority of early detection of albuminuria and prompt initiation of appropriate therapy to prevent the need for renal replacement treatment. Nonsteroidal Mineralocorticoid Receptor Antagonists (ns-MRAs), such as finerenone, represent a major advance in the management of DKD, demonstrating significant cardiorenal benefits, high receptor selectivity and a lower risk of hyperkalemia. These properties position finerenone as the third cornerstone in the treatment of CKD and DKD, following RAAS blockade and SGLT2 inhibition. Beyond the independent efficacy of these three pharmacological classes, the combination of finerenone and empagliflozin enhances cardiorenal protection, supporting a synergistic, early and comprehensive treatment approach. Finally, GLP-1 receptor agonists, particularly semaglutide, complete the puzzle of quadruple therapy in DKD, strengthening their role in patients with type 2 diabetes, CKD and a body mass index (BMI) >30kg/m². The central challenge in DKD lies not only in halting disease progression but in transforming its prognosis. Through an integrated cardiorenal approach-with therapies such as finerenone-we can turn the progression to ESKD into an opportunity for protection and renewed hope for our patients.
Figure 1:Management of Diabetic Kidney Disease (DKD). Adapted from reference [16].
References
- Hoogeveen EK (2022) The Epidemiology of diabetic kidney disease. Kidney Dial 2(3): 433-442.
- Brenner BM, Cooper ME, Zeeuw DD, Keane WF, Mitch WE, et al. (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345(12): 861-869.
- Büttner F, Barbosa CV, Lang H, Tian Z, Melk A, et al. (2023) Treatment of diabetic kidney disease. A network meta-analysis. PLoS One 18(11): e0293183.
- Heerspink HJL, Stefánsson BV, Correa RR, Chertow GM, Greene T, et al. (2020) Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383(15): 1436-1446.
- Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. (2023) Empagliflozin in patients with chronic kidney disease. N Engl J Med 388(2): 117-127.
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, et al. (2020) Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383(23): 2219-2229.
- Piko N, Bevc S, Hojs R, Ekart R (2024) Finerenone: From the mechanism of action to clinical use in kidney disease. Pharmaceuticals (Basel) 17(4): 418.
- Zhai S, Ma B, Chen W, Zhao Q (2024) A comprehensive review of finerenone-a third-generation non-steroidal mineralocorticoid receptor antagonist. Front Cardiovasc Med 11: 1476029.
- Naaman SC, Bakris GL (2023) Diabetic nephropathy: Update on pillars of therapy slowing progression. Diabetes Care 46(9): 1574-1586.
- Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, et al. (2021) Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 385(24): 2252-2263.
- Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, et al. (2022) Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur Heart J 43(6): 474-484.
- Kidney disease: Improving global outcomes (KDIGO) ADPKD Work Group (2025) KDIGO 2025 clinical practice guideline for the evaluation, management and treatment of autosomal dominant polycystic kidney disease (ADPKD). Kidney Int 107(2S): S1-S239.
- American Diabetes Association Professional Practice Committee (2025) 11. Chronic kidney disease and risk management: Standards of care in diabetes-2025. Diabetes Care 48(1 Suppl 1): S239-S251.
- Agarwal R, Green JB, Heerspink HJL, Mann JFE, McGill JB, et al. (2025) Finerenone with empagliflozin in chronic kidney disease and type 2 diabetes. N Engl J Med 393(6): 533-543.
- Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, et al. (2024) Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 391(2): 109-121.
- Zannad F, McGuire DK, Ortiz A (2025) Treatment strategies to reduce cardiovascular risk in persons with chronic kidney disease and type 2 diabetes. J Intern Med 297(5): 460-478.
© 2025 Divisón-Garrote JA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






