- Submissions

Full Text
Examines in Marine Biology & Oceanography
Shell Secrets of Tangkak Population: Microstructural and Elemental Insights into Trace Metal Pollution using Perna Viridis as a Coastal Biomonitor
Chee Kong Yap1*, Muhammad L Badamasi2, Kennedy A Aguol3, Rosimah Nulit1, Noraini A Bakar1, Wan M Syazwan1, Yoshifumi Horie4, Meng C Ong5,6, Mohamad S Ismail7, Ahmad D Setyawan8,9, Krishnan Kumar10 and Sarini a Wakid11
1Department of Biology, University Putra, Faculty of Science, Malaysia
2Biology Dept School of Secondary Education (Sciences), Federal College of Education, Nigeria
3Centre for the Promotion of Knowledge and Language Learning, PPIB, Jalan UMS, Universiti Malaysia Sabah, Malaysia
4Graduate School of Maritime Sciences, Faculty of Maritime Sciences, Kobe University, Japan
5Faculty of Science and Marine Environment, Universiti Malaysia Terengganu, Malaysia
6Ocean Pollution and Ecotoxicology (OPEC) Research Group, Universiti Malaysia Terengganu, Malaysia
7Fisheries Research Institute, Malaysia
8Department of Environmental Science, Faculty of Mathematics and Natural Sciences, Universitas Sebelas Maret, Indonesia
9Biodiversity Research Group, Universitas Sebelas Maret, Indonesia
10Faculty of Health and Life Sciences, INTI International University, Malaysia
11School of Biology, Universiti Teknologi MARA, Malaysia
*Corresponding author: Chee Kong Yap, Department of Biology, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
Submission: October 10, 2025;Published: December 17, 2025

ISSN 2578-031X Volume7 Issue 5
Abstract
This study investigates the microstructural integrity and elemental composition of the green-lipped mussel Perna viridis shells collected from Tangkak coastal waters, Johore, using Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy (SEM-EDS). Although the region is graded as a relatively less polluted zone, comprehensive analyses revealed significant alterations in the shell microstructure and the presence of trace metals such as Tin (Sn), Antimony (Sb), and Iodine (I). The nacreous layer had disorganized wavy lamellar structures, suggesting disturbance of biomineralization most probably from extended period of exposure to the surrounding environment. SEM-EDS spectra indicated uniform detection of Sb and Sn at all locations investigated, suggesting diffuse pollution and industrial discharge as causal factors. The findings provide proof that mussel shells are not only passive biological archives of the stresses in the environment but also acted as sensitive biomonitoring material of new metal pollutants in coastal biota. The study emphasizes the significance of microanalytical techniques could be applied to environmental monitoring and instigates heighten biomonitoring efforts in areas hitherto deemed ecologically stable. Focusing on Tangkak, the study broadens our understanding of coastal pollution processes as well as affirms the role of P. viridis shells as biomonitoring materials in the surveillance of marine ecosystem health.
Keywords:Perna viridis; Sem-eds; Tangkak; Trace metals; Biomonitoring
Introduction
Marine mussels have long been recognized as being useful for environmental monitoring due to the fact that they are sessile, abundant, and have a tendency to bioaccumulate contaminants from their surroundings. The shells of these organisms, which are predominantly composed of calcium carbonate in the form of aragonite, are long-term integrators of environmental conditions and bear a signature of trace element variation over time [1,2]. Their biomineralized tissues are particularly valuable for chronic trace metal exposure monitoring since they allow scientists to detect subtle signs of pollution even when water quality parameters are within the normal range [3,4] Mussel shells, in this context, provides as an excellent passive but effective tool for the reconstruction of pollution histories in coastal environments that have been impacted by industrial or domestic effluents. The uptake of trace metals such as Tin (Sn), Antimony (Sb), and Iodine (I) in the shells of bivalves has been of great concern due to their ecotoxicological and biomineralization-disrupting properties [5,6]. These could have originated from various anthropogenic sources, including the electronics, battery-manufacturing, glass, and metalfinishing industries, and agricultural industries and domestic wastewater [7,8].
Their presence in mussel shells shows the bioavailability of metals in aquatic systems and suggests the danger of sublethal effects to aquatic organisms. Specifically, studies have illustrated that metal-induced shell matrix changes have the potential to disrupt the organized crystal growth of aragonite layers, resulting in microstructural defects capable of compromising shell strength and integrity [9,10]. Scanning Electron Microscopy Coupled with Energy-Dispersive X-Ray Spectroscopy (SEM-EDS) has become a cornerstone method to investigate the surface morphology and elemental composition of mussel shells. The methods allow for the detection of heavy metals at microscale resolution and also provide further insights into the processes by which contaminants become integrated into the shell structure [11,12] Muscular morphological disruptions in the form of irregular layering, wavy structures, or granularity have been linked to exposure to impurities such as Sb and Sn, which alter the physicochemical environment of shell formation [13,14] Thus, the combination of SEM imaging and EDS elemental mapping furnishes a more comprehensive record of the history of exposure and environmental stressors affecting mussel coastal ecosystems. Even though coastal waters of Tangkak in the State of Johor Darul Takzim are assumed to be relatively unpolluted, there is a lack of microstructural and elemental data on resident Perna viridis populations. In the face of increasing industrialization and diffuse pollution sources, it is particularly necessary to reassess the environmental quality of such locales using biomonitoring tools. Therefore, the objective of this study is to examine the microstructural characteristics and elemental content of mussel shells collected from Tangkak through the use of SEM-EDS, with a particular emphasis on tracing the presence of trace metals and whether they can serve as early biomonitoring materials of anthropogenic pollution.
Materials and Methods
Figure 1:Scanning Electron Microscopy (SEM) and energy-dispersive x-ray spectroscopy.
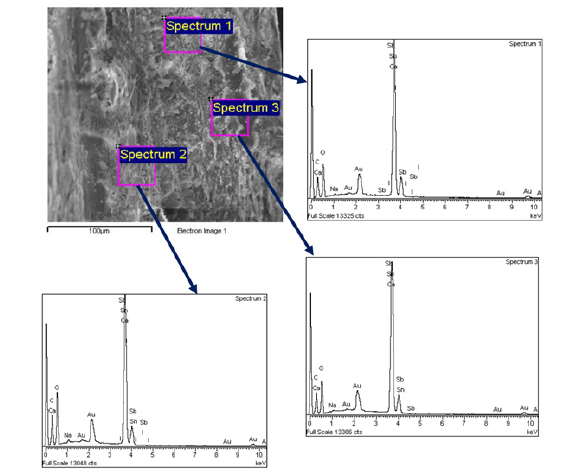
Tangkak in the State of Johor Darul Takzim is located at the south western coastal waters of Peninsular Malaysia (Figure 1), it yielded specimens of shells with continuous and discontinuous deformities. These deformities were found in the posterior end as well as, in some specimens, the dorsal side of the posterior shell field. Shells were washed in soapy tap water during collection to remove surface debris and organic matter. This was then followed by a single wash in double-distilled water. The washed shells were left to 5-days shaded indoors air-drying in order to avoid photodegradation due to direct sunlight exposure. Following dedication, all the shells were manually sectioned transversely across deformed regions with a handheld multipurpose jeweler’s alloy-steel hacksaw blade, and their outer shell surface uppermost in a bid to preserve the structural orientation. The resulting rough cross-sections were subsequently polished with 800Cw silicon-carbide waterproof abrasive paper to a suitable SEM surface for analysis.
In preparation for imaging and elemental analysis, prepared cross-sections were coated with a thin film of gold (Au) for surface conductivity purposes. The samples were then characterized using a JEOL JSM-6400 scanning electron microscope, located at the microscopy unit, Institute of Bio Science (IBS), Universiti Putra Malaysia (UPM). For each sample, SEM images were captured at three random points on the surface of cross-sections and photos were also captured at a ×1000 magnification level. One representative image at ×1000 magnification was captured from each shell. For each photo, three various EDX spectra (labelled as Spectrum 1, Spectrum 2 and Spectrum 3) were captured in order to describe the elemental composition of ultrastructure of the shell. The SEM-EDX results were then used to compare and validate for the presence of major and trace elements, making it possible to characterize the biomineral matrix and possible indication of contaminations. This was done following the procedure of analysis by Scimeca et al. for analysis of ultrastructural integrity and elemental uptake in bio-samples.
Result
The SEM-EDS was employed to investigate the surface morphology and elemental composition of P. viridis shells from Tangkak, Johor. The 1000× magnified shell samples revealed extremely intricate and irregular structural features throughout the cross-section of the nacreous. Depiction in Figure 1, is the micrograph showing an amorphous and wavy distribution of biomineralized layers, indicating possible deviations of the usual aragonite lamellar arrangement. Three regions were selected for EDS analysis-Spectrum 1, Spectrum 2 and Spectrum 3-each having varying microstructural heterogeneity in the same shell. (EDS) analysis of the cross-sectional nacreous layer of Perna viridis shells collected from Tangkak, Johor. The SEM micrograph (top left) at 1000× magnification displays the fine microstructural features of the shell’s aragonite matrix, including visible lamellar and granular arrangements. Three specific sites were selected for point EDS analysis-designated as Spectrum 1, Spectrum 2, and Spectrum 3-highlighted with magenta boxes and labelled accordingly. The corresponding EDS spectra for each spot are shown adjacent to the SEM image and connected via directional arrows. Elemental composition (in % by weight) for all the spectra is provided in Table 1. The main elements found in all the spectra were Oxygen (O), Calcium (Ca), and Carbon (C), which are consistent with the aragonitic nature of the shell matrix. Oxygen was the most common element, ranging from 47.88% (Spectrum 3) to 53.18% (Spectrum 2). Carbon content was also varied slightly between 12.72% (Spectrum 1) and 15.71% (Spectrum 2). The high calcium content, particularly in Spectrum 1 (35.75%) and Spectrum 3 (35.28%), suggests the dominant CaCO₃ (Calcium Carbonate) composition of the shell common to different biomineralized marine bivalves. Spectrum 2 contained the lowest value of calcium at 27.78%, which might reflect localized deficiency or structural anomalies.
Table 1:Summary of the elemental composition (%) for each spectrum at Tangkak population.

From the trace elements, Sodium (Na) consistently occurred at low concentrations (0.43-0.69%), perhaps through ionic exchange or external exposure to domestic effluence and saline waters. Of particular note is the occurrence of potentially toxic elements: Tin (Sn), Antimony (Sb), and Iodine (I). Sn contents ranged from 0.70% to 0.92%, with the highest content in Spectrum 3. Sb was highly variable, with the highest content similarly occurring in Spectrum 3 (1.68%) and the lowest content in Spectrum 2 (1.26%). Iodine was present in all three spectra at levels that were similar (0.66-0.67%) and are of concern for the potential input of iodinebased disinfectants, pharmaceuticals, or fertilizer runoff into the aquatic system. The simultaneous identification of Sb and Sn-both industrial contaminants-across the whole area of the shell suggests that despite the classification of Tangkak as a comparatively clean location, the mussels may have been exposed to trace amounts of industrial effluents. The presence of Au peak in the EDS spectra is because of sputter-coating during SEM sample preparation and is not due to the environment.
Discussion
Structural disturbance in the mussel shell nacreous layer
The SEM-EDS micrograph (Figure 1) shows wavy, clumped, and irregular microstructures in the nacreous layer of Perna viridis shells collected from Tangkak. The nacreous layer of bivalves typically exhibits a highly organized, lamellar structure composed primarily of aragonite, which provides both mechanical strength and biomineral integrity [15,16] The structural abnormalities observed in this study-irregular layering, surface granulation, and potential fractures-suggest that the biomineralization process in these mussels may have already been compromised. These microstructural changes can be interpreted as stress responses to chronic environmental exposure to contaminants, particularly trace metals. Similar morphological disturbances have been recorded in Mytilus galloprovincialis (Mediterranean mussel/ Black Sea coastal mussel) and Mytilus coruscus (Korean mussel/hard-shelled mussel) when exposed to pollutants, such as nano-TiO₂ and acidified seawater, resulting in altered crystal orientation and reduced shell robustness [14,17]. Environmental stressors like elevated metal concentrations can disrupt the normal crystallization and assembly of aragonite layers, which is a hallmark of shell deterioration and vulnerability [9,10] In this context, the visible microstructural anomalies serve not only as an indication of on-going physiological stress but also highlight the mussel’s sensitivity to environmental changes. This reinforces the role of P. viridis shell as a valuable biomonitoring material of biomineralization disruption caused by coastal contamination [11,18]. Furthermore, these shell deformities support the idea of cumulative exposure, where the bivalve’s ability to maintain shell integrity is progressively compromised over time. This phenomenon underscores the importance of shell morphology studies, not just for understanding biomineralization under stress, but also for monitoring latent pollution that may not be immediately detectable through water sampling alone.
Bioaccumulation of trace elements: Environmental and industrial implications
The SEM-EDS spectra and corresponding elemental analysis (Table 1) revealed significant accumulation of trace elements such as Sn, Sb, and I within the shell matrix. Sn was found in the range of 0.70-0.92%, while Sb ranged from 1.26% to 1.68%, both of which align with previous reports of industrial inputs along the West Coast of Peninsular Malaysia, particularly from electronics manufacturing, textile dyeing, and metal-plating industries [3,7]. The incorporation of Sb is particularly concerning due to its known affinity for bioaccumulation even at low environmental concentrations and its adverse effect on shell formation and calcification processes [5,9]. The consistent detection of iodine in all sampling spectra (0.66-0.67%) could originate from agricultural fertilizers, aquaculture additives, or iodine-based disinfectants frequently used in healthcare and sanitation [8]. While iodine is essential in trace amounts, elevated concentrations can indicate anthropogenic intrusion into marine systems. These contaminants are persistent, bioavailable, and capable of integrating into shell matrices, which validates their inclusion in ecotoxicological assessments [6,19]. The presence of these elements in a site categorized as “relatively unpolluted” suggests that diffuse and long-range transportation of pollutants are plausible contamination pathways [20]. Supporting evidence from studies in the Crimean Peninsula and the Black Sea also highlights the widespread nature of such trace element inputs in Mytilus galloprovincialis (Mediterranean mussel/ Black Sea coastal mussel) [4,21]. These findings question the reliability of traditional water-based assessments and elevate the importance of biomonitoring frameworks that incorporate bivalve shell analysis as a long-term exposure biological archive.
Mussel shells as coastal pollution biomarkers
The elemental profile observed in the P. viridis shells from Tangkak substantiates their role as passive biomonitoring materials of environmental quality. Unlike soft tissues that respond to short-term exposure, shells reflect cumulative assimilation of environmental signals over extended periods, offering a more integrative view of ecological stressors [1,22]. The dominant presence of aragoniteassociated elements-calcium (27.78-35.75%), carbon (12.72- 15.71%), and oxygen (47.88-53.18%)-aligns with healthy shell formation patterns reported in earlier biomineralization studies [23,24]. However, deviations in sodium content and the presence of trace metals reveal that external environmental factors modulate ionic incorporation during shell formation. Such variations have been linked to site-specific pollution gradients, seasonal dynamics, and hydrological variability that influence bioavailability and uptake [25,26]. Furthermore, elemental mapping and multivariate statistical techniques have demonstrated that shell chemical profiles can be effectively used for geographic traceability and pollution source discrimination [2,12]. When combined with observed shell deformities, the altered elemental signatures act as early warning indicators of sublethal metal stress and potential ecosystem degradation [27,28]. Incorporating mussel shell analysis into coastal environmental monitoring is not only cost-effective but also non-invasive and highly informative. It enables long-term assessment of contaminant bioavailability and ecosystem integrity in dynamic marine habitats. Thus, the findings of this study further advocate for the inclusion of biomineral indicators like P. viridis shells in regular coastal pollution surveillance programs, especially in semi-urban and the industrialization of coastal zones like Tangkak.
Conclusion
This study highlights the promise of P. viridis shells as valuable biomonitoring materials for assessing trace metal contamination in coastal environments. Through SEM-EDS analysis, crosssectional nacreous layers of shells collected in Tangkak, which was previously considered a relatively unspoiled region, did exhibit microstructural deformation and quantifiable amounts of industrially associated metals such as Sn, Sb, and I. The wavy and asymmetrical lamellar texture observed on SEM suggests possible disruptions in biomineralization events due to prolonged exposure to environmental stress factors. The presence of Sb and Sn at significant concentrations in all spectra is evidence for hypothesis of extensive anthropogenic contamination with diffusion into coastal waters, e.g., through industrial effluents, electronics and semiconductors factories, domestic sewage and agricultural runoff. These findings not only validate the utility of mussel shells as long-term, passive marine quality monitors but also underscore the need for continued monitoring in areas considered to be pristine. Structural and chemical determinations combined result in enhanced understanding of sublethal effects of metal exposures on bivalves from marine habitats and of precursors to deterioration of ecosystems. Subsequent research would be well advised to include comparative biomonitoring and continuous surveillance at multiple locations, time-series sampling, and incorporation with water and sediment analysis in order to provide a better define contaminant pathways. Ultimately, coastal health is a matter of preempting biomonitoring with evidence-based methods like those represented here.
References
- Schöne BR, Krause RA (2016) Retrospective environmental biomonitoring-mussel watch expanded. Global and Planetary Change 144: 228-251.
- Shirai K, Takahata N, Yamamoto H, SanoY, Omata T, et al. (2008) Novel analytical approach to bivalve shell biogeochemistry: A case study of hydrothermal mussel shell. Geochemical Journal 42(4): 413-420.
- Ng YJ, Yap CK, Zakaria MP, Tan SG, Aris AZ (2013) Trace metals in the shells of mussels perna viridis transplanted from polluted to relatively unpolluted sites in the straits of Johore: Shells as biomonitoring materials. Asian Journal of Microbiology, Biotechnology and Environmental Sciences 15(1): 5-8.
- Zakharikhina L, Rudev P, Paltseva A (2022) Chemical composition and morphology of the Mediterranean mussel black sea coast of Russia. Marine Pollution Bulletin 179: 113692.
- Fent K (2003) Ecotoxicological problems associated with contaminated sites. Toxicology Letters 140-141: 353-365.
- Antunes SC, Pereira R, Marques SM, Gonçalves F, Castro BB (2011) Impaired microbial activity caused by metal pollution: A field study in a deactivated uranium mining area. Science of the Total Environment 410-411: 87-95.
- Galán E, González, I, Romero A, Aparicio P (2014) A methodological approach to estimate the geogenic contribution in soils potentially polluted by trace elements. Journal of Soils and Sediments 14(7): 1221-1233.
- Mafumo N, Bezuidt OKI, Roux IW, Makhalanyane TP (2023) Crass phage may be viable markers of contamination in pristine and contaminated river water. MSystems 8(1): e0128222.
- Stewart BD, Jenkins SR, Boig C, Kröger R, Sinfield C, et al. (2021) Metal pollution as a potential threat to shell strength and survival in marine bivalves. Science of the Total Environment 770(Pt 1): 143019.
- Thomsen J, Ramesh K, Sanders T, Melzner F (2018) Calcification in a marginal sea-Influence of seawater [Ca²⁺] and carbonate chemistry on bivalve shell formation. Biogeosciences 15(4): 1067-1080.
- Mirapeix J, Mateos A, Escárzaga RG, Cobo A, Zugasti IG, et al. (2025) Virtual sampling: Archaeological implications of a new technique for elemental mapping of Mg/Ca ratios in marine mollusc shells. Journal of Archaeological Science 173: 106123.
- Forleo T, Zappi A, Melucci D, Piersanti A, Ciriaci M, et al. (2021) Inorganic elements in Mytilus galloprovincialis shells: Geographic traceability by multivariate analysis of ICP-MS data. Molecules 26(9): 2634.
- Kocabaş FK, Kocabaş M, Çanakçi A, Karabacak AH (2023) Mechanical property and structural-elemental analysis of marine bivalve mollusc shells: Cerastoderma edule, Chamelea gallina, Donax trunculus, Ruditapes decussatus. International Aquatic Research 15(1): 39-50.
- Zhao X, Han Y, Chen B, Liu G, Qu K (2020) CO₂-driven ocean acidification weakens mussel shell defense capacity and induces global molecular compensatory responses. Chemosphere 243: 125415.
- Ismail R, Cionita T, Shing WL, Endot NA, Fitriyana FD, et al. (2022) Synthesis and characterization of calcium carbonate obtained from green mussel and crab shells as a biomaterial’s candidate. Materials 15(16): 5712.
- Kobelja K, Nemet I, Župan I, Rončević S, Čulin J (2016) Elemental profiling of Noah’s Ark shell (Arca noae, Linnaeus, 1758) by plasma optical spectrometry and chemometric tools. Journal of Trace Elements in Medicine and Biology 38: 157-164.
- Huang X, Lin D, Ning K, Wang Y, Sui Y, et al. (2016) Hemocyte responses of the thick shell mussel mytilus coruscus exposed to nano-TiO₂ and seawater acidification. Aquatic Toxicology 180: 1-10.
- Hartmann JT, Beggel S, Auerswald K, Geist J, Stoeckle B (2016) Establishing mussel behavior as a biomarker in ecotoxicology. Aquatic Toxicology 170: 279-288.
- Seoane G, Aboal R, Boquete JR, Fernández JA (2020) Phenotypic differences in heavy metal accumulation in populations of the brown macroalgae Fucus vesiculosus: A transplantation experiment. Ecological Indicators 111: 105978.
- Fent K (2004) Ecotoxicological effects at contaminated sites. Toxicology 205(3): 223-240.
- Nekhoroshkov P, Zinicovscaia I, Vergel K, Kravtsova A, Grozdov D, et al. (2022) Macro-and microelements and radio nuclides in the mussel Mytilus galloprovincialis from recreational and harbor sites of the Crimean Peninsula (The Black Sea). Hydrobiology 1(3): 304-316.
- Motrescu I, Calistru AE, Jitareanu G, Miron LD (2020) Monitoring the environmental quality of marine waters through the analysis of biomineralization in bivalve shells. Lecture Notes in Networks and Systems 91: 269-277.
- Piórewicz AP, Kukliński P, Strekopytov S, Iglikowska A, Williams EH, et al. (2017) Size effect on the mineralogy and chemistry of Mytilus trossulus shells from the southern Baltic Sea: Implications for environmental monitoring. Environmental Monitoring and Assessment 189(4): 197.
- Hopper GW, Dickinson GK, Atkinson CL (2021) Associations among elements in freshwater mussel shells (Unionidae) and their relation to morphology and life history. Freshwater Biology 66(10): 2149-2161.
- Grzywacz B, Śliwa W, Banach E, Pyza EZ (2012) Genetic variability and changes of elemental concentrations in cells of tetrix tenuicornis (Orthoptera: Tetrigidae) from polluted and unpolluted areas. Folia Biologica 60(1-2): 17-25.
- Lacroix C, Richard G, Seguineau C, Auffret M, Guyomarch J, et al. (2015) Active and passive biomonitoring suggest metabolic adaptation in blue mussels (Mytilus spp.) chronically exposed to moderate contamination in Brest harbor, (France). Aquatic Toxicology 167: 117-129.
- Štambuk A, Šrut M, Šatović Z, Klobučar GIV, Tkalec M (2013) Gene flow vs. pollution pressure: Genetic diversity of mytilus galloprovincialis in eastern adriatic. Aquatic Toxicology 136-137: 22-31.
- Yawetz A, Fishelson L, Bresler V, Manelis R (2010) Comparison of the effects of pollution on the marine bivalve Donax trunculus in the vicinity of polluted sites with specimens from a clean reference site (Mediterranean Sea). Marine Pollution Bulletin 60(2): 225-229.
© 2025 Chee Kong Yap*. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






