- Submissions

Full Text
Environmental Analysis & Ecology Studies
Sustainable Ecotechnological Remediation and Recovery of Chromium from Wastewater Using the Floating Aquatic Plant Azolla pinnata
K Usharani1,2* and PJ Nivetha1
1Department of Environmental Science, PSG College of Arts and Science, India
2Department of Civil and Environmental Engineering, Sao Paulo State University Julio De Mesquita Filho, UNESP, Bauru, SP, Brazil
*Corresponding author:K Usharani, Department of Civil and Environmental Engineering, School of Engineering, Sao Paulo State University Julio De Mesquita Filho, UNESP, Bauru, Brazil
Submission: October 17, 2025; Published: December 19, 2025

ISSN 2578-0336 Volume13 Issue 3
Abstract
Phytotechnology utilizes aquatic macrophytes and their microbiomes to remediate chromium-contaminated waters. This study evaluates Azolla pinnata, a free-floating fern, for its potential in sustainable remediation and recovery of Cr(VI) from synthetic wastewater. Results show successful removal of hexavalent chromium through rhizofiltration, where Azolla and algae synergistically reduce chromium concentration. Batch tests at varying initial Cr(VI) concentrations (5, 10, 25, 40, and 50ppm) using 8g of fresh Azolla biomass indicated significant chromium absorption, evidenced by a dark brown coloration. The experiment lasted 14 days, with chromium quantified via the Diphenylcarbazide (DPC) assay and measurements taken spectrophotometrically at 540nm. The findings confirm the effectiveness of this phytoremediation process and highlight the potential of aquatic plants in addressing environmental challenges while promoting a cleaner, healthier earth.
Keywords:Phytoremediation; Chromium; Azolla pinnata; Bioremoval
Introduction
Heavy metal pollution-particularly chromium-poses a global threat. Conventional treatments (ion exchange, membranes, reverse osmosis, precipitation) can be effective but cost-intensive, whereas phytoremediation offers a low-cost, nature-based solution. Azolla pinnata, which hosts Nostoc azollae, grows rapidly and is easily harvested, enabling rhizofiltration and biosorption of dissolved metals. However, the development of improved solutions is limited, particularly in developing countries, due to economic constraints and the high costs of acquiring quality equipment. Phytoremediation is a cost-effective green technology that has proven effective in various water systems, such as dams and lakes. This bioremediation process utilizes different plant species to remove, transfer, stabilize, or eliminate heavy metal deposits in water. One such plant, Azolla pinnata, is a small free-floating aquatic macrophyte that effectively absorbs heavy metals from concentrated sources.
Azolla thrives in symbiosis with cyanobacteria, which live within the cavities of its fronds. Its rapid growth rate, capable of doubling its biomass in just 2 to 4 days, along with its ability to float, makes it easy to harvest and facilitates the removal of heavy metals from contaminated water. Phytoremediation leverages these plants to degrade, stabilize, digest, or eliminate toxins and other chemical pollutants. The aquatic plants always have an extensive system of roots that helps them and makes them the primary route for the accumulation of contaminants in their roots and shoots. The aquatic free-floating plants have been revealed to be a principled prototype for ecotoxicological studies, and they have the competence to bioaccumulate or biosorb nutrients and metals [1-3].
The benefits of using plants for this purpose include economic advantages, effective harvest management, and the potential utilization of harvested products. Azolla is particularly promising for the removal of metal ions. Chromium, when present in high concentrations, poses toxic risks to humans, animals, and the environment. Specific tests for chromium have been conducted and stored for laboratory studies on phytoremediation. The aquatic plant Azolla is currently being investigated for its effectiveness in removing chromium from solutions at various concentrations, with the levels of chromium analyzed using UV spectrophotometer readings at 540nm. The nearby electroplating industry produces chromium alloys at high levels, making this study crucial for reducing chromium concentrations to safer levels.
Materials and Methods
Plant acquisition and acclimatization
Azolla pinnata was collected from the Azolla growing bed near the Horticulture Centre for the experiment. The plants were washed with running water to remove any mud particles from the roots. To support adaptation to the experimental conditions and to produce substantial biomass, the plants were grown in a plastic tray filled with tap water for approximately 15 days.
Chromium standards, controls, and analytical method
Chromium was quantified via the Diphenylcarbazide (DPC) colorimetric assay (λ=540nm), with acidification by H2SO4 and calibration curves from standards and blanks; measurements taken at 5min after reagent addition. A few drops of concentrated sulfuric acid were added to establish acidic conditions. The mixture was then diluted to a final volume of 50mL using distilled water. After 5 minutes, 1,5-Diphenylcarbazide (DPC) reacts selectively with Cr(VI) to afford a complex with a purplish red color. The intensity of the resulting color was measured at 540nm using a UV spectrophotometer.
Estimation of the concentration of chromium after phytoremediation treatment using Azolla pinnata
Stock solutions of potassium dichromate are prepared in water at known concentrations and quantities. Aquatic plant samples of Azolla are weighed and placed into 12-liter plastic containers filled with a specific volume and concentration of the metallic compound in water. The experimental setup is maintained under partially covered conditions. To account for evaporative losses, distilled water is added every third day to maintain a consistent water level. Water samples from the containers are collected every seventh day for analysis. The condition of the plants is visually assessed during these intervals. After 28 days, the plants are removed, weighed, and then returned to their original water source. To analyze the concentration of metal ions in the water samples collected weekly, and analyzed by a UV spectrophotometer. Based on the absorbance readings of the known solution samples, a standard graph is plotted to illustrate the relationship between absorbance and concentration. This graph is then referenced to determine the concentration of the water samples collected from the containers over time [4].
The samples were allowed to cool, and the absorbance in terms of Optical Density (OD) at 540nm was measured using a UVVisible spectrophotometer. The initial and residual concentration of chromium in terms of Optical Density (OD) value was measured using a UV-Visible spectrophotometer (UV-2600 series SHIMADZU).
From this value, the percentage chromium reduction or removal was calculated.
The percentage Chromium removal efficiency (R) was
calculated using the formula,
R = (I − F) / I × 100
Where I and F are the Initial and Final concentrations of Chromium, respectively.
The Chromium Removal Efficiency (%R): This measures the
percentage of the contaminant removed from the solution/soil
relative to the initial amount. It is calculated using the standard
formula:
(%R) = (Ci −Cf ) / Ci × 100
where (Ci) is the initial concentration and (Cf) is the final concentration.
An efficiency value of 100% was obtained when no Chromium appeared in the treated water sample (i.e., Final concentration=0). The fresh and dried biomass of A. pinnata plants was measured. Growth of the plant biomass (A. pinnata) was measured in terms of dry weight by the gravimetric method [2,3,5,6].
Estimation of pH after phytoremediation using Azolla pinnata
The pH change in treated wastewater at different concentrations and with varying time intervals, as affected by free-floating aquatic plants A. pinnata, was estimated using a digital pH meter (Scientific Tech, Advanced pH meter, model ST-2002).
Estimation of biomass after phytoremediation treatment
On the 14th day of the experiment, the fresh and dried biomass of A. pinnata plants was measured. Each replicate was harvested, and the plants were rinsed with distilled water to eliminate any ions adhering to their surfaces. The plants were then blotted with filter paper to remove excess water. After measuring the fresh biomass, the samples were dried in an oven at 80 °C for 48 hours. The dry weights were subsequently recorded [7].
Estimation of chlorophyll after phytoremediation treatment
Approximately 1 gram of finely chopped plant material was mixed with 20 milliliters of 80% acetone and ground in a clean mortar. The mixture was then centrifuged at 500RPM for 5 minutes, and the supernatant was transferred to a 100-milliliter volumetric flask. This centrifugation process continued until the residue became colorless. The solution was then diluted to a final volume of 100 milliliters with 80% acetone, and the absorbance was measured spectrophotometrically at wavelengths of 664nm and 647nm.
Result and Discussion
Effect of chromium after phytoremediation treatment
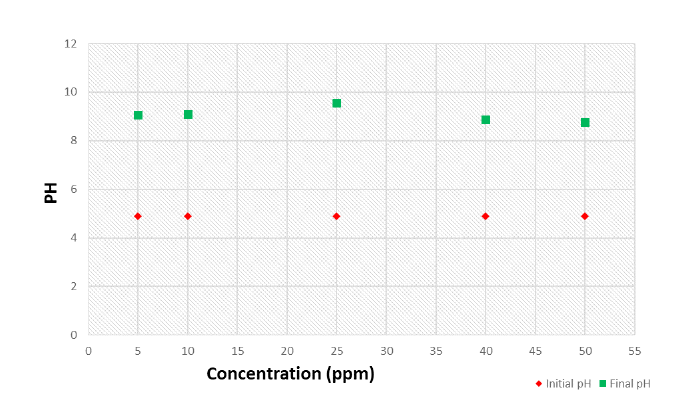
The results indicate that phytoremediation is effective at a wavelength of 540nm, as measured using a UV spectrophotometer. When compared to standard chromium levels, Azolla pinnata effectively reduces chromium concentrations after the phytoremediation process. Based on these observations, it can be concluded that Azolla pinnata can thrive in chromium concentrations of less than 5ppm and can tolerate a maximum concentration of 50ppm (Figure 1A&1B). After seven days of treatment with Azolla pinnata, the percentage removal of chromium reached a maximum of 94.8% at a 5ppm chromium concentration and a minimum of 16.5% at a 50ppm concentration (Figure 2A). The effects of chromium through sustainable ecological phytoremediation after seven days are shown in Figure 3. After fourteen days of treatment, the percentage removal of chromium by Azolla pinnata increased, achieving a maximum bioremoval of 97.3% at 5ppm chromium concentration and a minimum of 16.7% at 50ppm (Figure 2B). The effects of chromium via sustainable ecological phytoremediation after fourteen days are illustrated in Figure 2. Compared to the results from day seven, Azolla pinnata significantly reduces chromium concentration after fourteen days of phytoremediation. These observations suggest that the reduction in chromium concentration is greater at 5ppm compared to the measurements taken on the seventh day. Removal efficiency decreased as initial Cr(VI) concentration increased, consistent with sorption site saturation and stress effects; at 40-50ppm, removal was notably reduced (≈16-43%). Additionally, Figure 3A&3B illustrates that on the 7th and 14th day, the leaves of Azolla pinnata remained fresh at a concentration of 5ppm; however, at higher concentrations, the leaves became completely dried out due to the stress caused by chromium toxicity. The highest recorded chromium removal rate was 97.34%. It is evident that as the concentration of chromium increases, the percentage of removal decreases. Figure 4 illustrates the impact of pH on chromium levels following phytoremediation, measured on the 14th day. Initially, the pH was acidic; however, after phytoremediation, it shifted to an alkaline state. According to Lindberg and Wingstrand (1985), plants can alter the pH of the surrounding water through their roots, which may help them cope with stress from heavy metals. Specifically, the roots significantly raised the rhizosphere pH under stress, especially when chromium was present in the solution. This shows the effects of chromium on biomass content after 14 days of phytoremediation.
Figure 1:Spectral observation of Chromium removal at 540nm (A) after the 7th day and (B) 14th day.
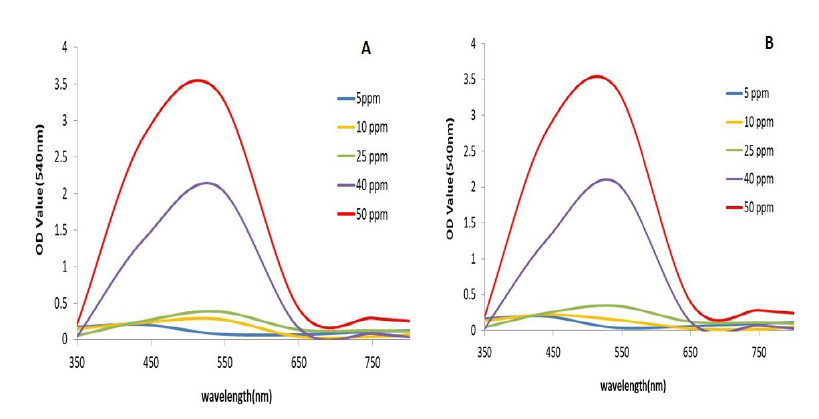
Figure 2:The percentage removal of chromium by Azolla pinnata (A) after the 7th day and (B) 14th day.
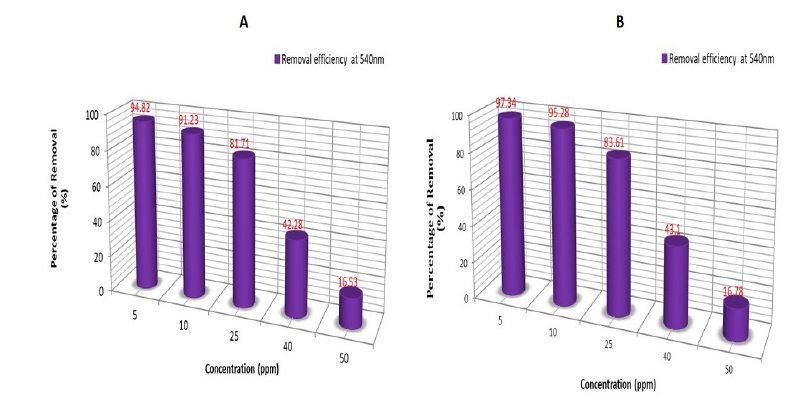
Figure 3:Effect of Chromium Phytoremediation by Azolla pinnata treatment after the (A) 7th day and (B) 14th day.
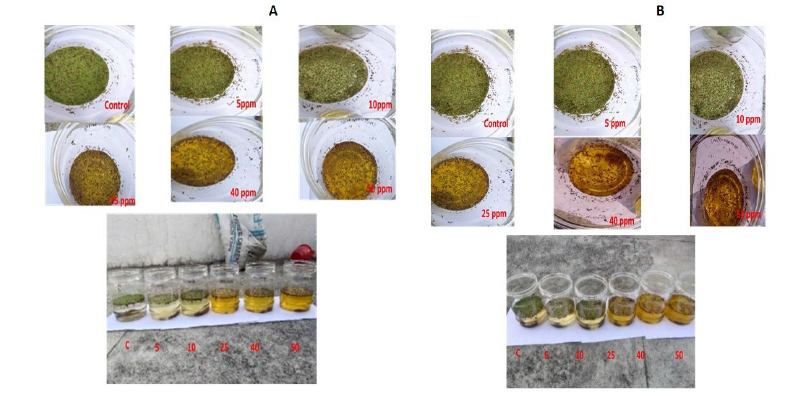
Figure 4:Effect of pH of the chromium after phytoremediation by Azolla pinnata treatment on the 14th day.
The growth rate of Azolla pinnata is negatively impacted by chromium stress. According to the analysis, there is a significant increase in the percentage decrease of fresh weight, as well as a notable reduction in the dry-to-fresh weight ratio at higher chromium concentrations. The maximum percentage decrease in fresh weight was observed with a 50ppm chromium solution after 14 days, suggesting that Azolla pinnata may accumulate chromium. Additionally, a higher dry weight-to-fresh weight ratio was noted at the lower concentration of 5ppm during the same 14-day period.
Figure 5 illustrates the impact of chromium on total chlorophyll content following phytoremediation over a period of 14 days. Chlorophyll content was assessed using acetone extracts, in accordance with the Arnon method (1949). The results indicate a decreasing trend in chlorophyll A content as chromium concentrations increased. In contrast, chlorophyll B and total chlorophyll contents showed an increasing trend; however, these overall values still declined with higher chromium concentrations. Elevated levels of chromium toxicity negatively affect photosynthesis. The reduction in chlorophyll content may be attributed to decreased enzymatic activity of protochlorophyllide reductase. Additionally, the lowered levels of photosynthetic pigments in plants could be linked to lipid peroxidation occurring in the chloroplast membranes.
Figure 5:Effect of chromium after phytoremediation by Azolla pinnata on Total Chlorophyll content after treatment on the 14th day
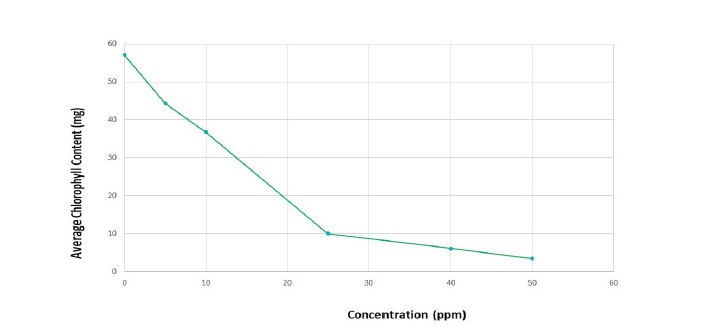
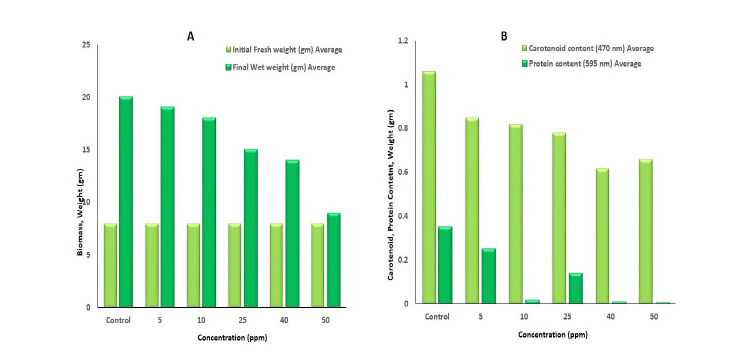
Figure 6A&6B illustrates the impact of chromium on carotenoid and protein content after 14 days of phytoremediation. It shows that as chromium concentration increases, carotenoid content decreases. According to Hou et al. (2007), carotenoids are nonenzymatic antioxidant pigments that protect cells from Reactive Oxygen Species (ROS) under chromium stress. The decline in carotenoid content can be attributed to metal toxicity, which may hinder the formation of photosynthetic pigments. This explains the observed reduction in photosynthetic pigments with increasing chromium concentration. Moreover, protein content also diminishes as chromium levels rise. Specifically, Azolla pinnata shows a reduction in protein content at higher chromium concentrations.
Figure 6:Effect of chromium after phytoremediation by Azolla pinnata on (A) Chlorophyll, (B) Carotenoid, and Protein content after treatment on the 14th day.
Phytotechnology harnesses aquatic macrophytes and their microbiomes to remediate chromium-contaminated waters. This study evaluates Azolla pinnata as a free-floating fern for sustainable remediation and potential recovery of Cr(VI) from synthetic wastewater. This study decisively demonstrates the successful removal of hexavalent chromium from contaminated synthetic wastewater using Azolla pinnata, highlighting a healthy, sustainable ecotechnology approach that capitalizes on the synergistic effects of both Azolla and algae. This innovative method, known as phytotechnology, encompasses processes such as bio-removal, bioreduction, and biodegradation. It prominently features rhizofiltration, where free-floating aquatic plants, such as Azolla pinnata, play a crucial role in pollutant removal. The combined action of the algae and Azolla leads to a significant reduction in chromium concentration through rhizofiltration, as these aquatic plants effectively adsorb pollutants from their environment.
As noted by Mishra et al., this decline can be linked to lower protein levels in aquatic macrophytes, as well as increased protein degradation resulting from metabolic processes. Chromium may disrupt the balance of free nucleotides and RNA, leading to reduced RNA biosynthesis, which could explain the decrease in protein synthesis. Azolla pinnata possesses an exceptional ability to hyperaccumulate heavy metals from contaminated water bodies (Wagnar, 1997). Ex situ research conducted by Salt et al. (1995), Bennicelli et al. (2004), Jangwattana (2010), Sood et al. (2011), Deval et al. (2012), Moradi et al. (2013), Sufian et al. [8], and Thayapara et al. [9] has demonstrated the various capacities of A. pinnata to uptake and retain different heavy metal ions. These findings highlight A. pinnata’s potential for the phytoremediation of heavy metal-polluted water reservoirs. The nearby electroplating industry discharges chromium, a heavy metal, into water bodies. The dried biomass of A. pinnata can be easily transported to recycling sites for heavy metal recovery (Sood, 2011). The study of aquatic free-floating plants, including L. minor, has been revealed to be a noble model for ecotoxicological studies, and it can bioaccumulate, or biosorption of nutrients and metals, was reported earlier [1-3].
This study confirms the efficacy of this phytoremediation treatment in removing heavy metals. Additionally, it is suggested that the 33 artificial systems integrated into the lake, planted with A. pinnata, can effectively function as biological filters to remove heavy metal ions from industrial discharges. Implementing such filter systems would ensure that industrial pollutants are captured and eliminated before contaminating public water bodies [10-15]. The results unequivocally demonstrated a significant reduction in total chromium concentration in the samples compared to the controls, confirming the effectiveness of the phytoremediation process. The aquatic, free-floating plants serve as a principled model for ecotoxicological studies, possessing the ability to bioaccumulate or biosorb nutrients and metals, thereby supporting sustainable development [16-21].
Conclusion
The primary characteristics of macrophytes that exhibit strong phytoremediation abilities include rapid growth rates, high biomass, and significant adaptability to a variety of environmental conditions. A notable example is the endosymbiont Nostoc azollae found in Azolla pinnata, which plays a crucial role in assimilating atmospheric nitrogen, enhancing the plant’s overall growth. This feature contributes to the vigorous reproduction of the water fern in irrigated environments. Additionally, the free-floating nature of A. pinnata and similar macrophytes simplifies the harvesting process. Their high-water content in fresh biomass, ranging from 90% to 94%, significantly reduces volume when dried, mitigating disposal challenges for the harvested material. Furthermore, A. pinnata demonstrates an impressive ability to thrive in highly polluted waters with varying pH levels, temperatures, and salinity, making it particularly suitable for phytoremediation applications. This study concludes by advocating for A. pinnata as a viable phytoremediation agent capable of addressing heavy metal contamination in public dams and waterways. Its delicate structure is especially beneficial for use in industrial ponds and rice paddies, where pollution from waste disposal and agricultural chemicals is a significant concern. Harvesting the heavy metal-rich water ferns can be done mechanically or manually, facilitating the chemical extraction of heavy metals, such as chromium, for multiple recycling uses. Although A. pinnata may not serve as a complete solution to heavy metal pollution, it provides a promising approach to restoring water bodies contaminated with specific heavy metals by capturing them within the plant’s tissues. Azolla-based phytotechnology provides a pragmatic pathway to mitigate chromium pollution and support resource recovery; field deployment should pair with upstream source control and routine monitoring. This sustainable remediation approach not only addresses critical environmental challenges but also showcases the remarkable potential of leveraging natural solutions for a cleaner, healthier earth.
References
- Usharani K, Arunkumar V (2023) Bioremoval and resource recovery of nutrients by phytoremediation using aquatic free-floating plants Lemna minor. Ukrainian Journal of Ecology 13(5): 13-27.
- Usharani K, Keerthi KV (2020) Nitrate bioremoval by phytotechnology using utricularia Aurea collected from eutrophic lake of theerthamkara, Kerala, India. J Poll 6(1): 149-157.
- Usharani K, Divya K, Sruthilaya K (2020) Combined effect of nitrate bioremoval by aquatic free-floating plant and association with filamentous Cyanobacteria. Austin Environ Sci 5(1): 1043.
- Shekhar P, Prashik G (2016) Phytoremediation studies for removal of copper & chromium using azolla pinnata and water hyacinth. International Journal of Innovative Research in Science, Engineering and Technology 5(5): 7078-7083
- Usharani K, Muthuchamy M, Perumalsamy L (2011) Biological removal of phosphate from synthetic wastewater using bacterial consortium. Iran J Biotechnol 9(1): 37-49.
- Usharani K, Sruthilaya K, Divya K (2017) Determination of nitrate utilization efficiency of selective strain of bacillus sp. isolated from Eutrophic Lake, Theerthakara, Kasaragod Kerala. J Poll 3(1): 55-67.
- Mandakini U, Bandara N, Gunawardana D (2016) A study on the phytoremediation potential of Azolla pinnata under laboratory conditions. JTFE 6 (1): 01-49.
- Sufian J, Golchin A, Avanes A, Moradi S (2013) Potentials of azolla (Azollacaroliniana) for uptake of arsenic from contaminated waters with different levels of salinity. International Journal of Agriculture and Crop Sciences 6(12): 778-783.
- Thayapara M, Iqbal S, Chathuranga PKD, Iqbal MCM (2013) Rhizofiltration of pb by Azolla pinnata. International Journal of Environmental Sciences 3(6): 1811-1821.
- Annie MPA, Gilbert CS (2013) Phytoremediation: A green technology to remove environmental pollutants. American Journal of Climate Change 2(1): 71-86.
- Abdel WR, Lubberding HJ, Alaerts GJ (1995) Copper and chromium (III) uptake by duckweed, water science and technology. Water Science and Technology 32(11): 105-110.
- Gandhi N, Sirisha D, Chandra SKB (2013) Phytoremediation of chromium and fluoride in industrial waste water by using aquatic plant ipomoea aquatica. SPJPBS 1: 001-004.
- Malairajan S, Alemayehu AM, Vinodhini SR (2007) Studies on the removal of hexavalent chromium from industrial wastewater by using biomaterials. EJEAFChe 6(11): 2557-2564.
- Nelson M, Marta M, Elena M (2006) Phytoremediation and phytotechnologies: A review for the present and the future. Soil and Water Pollution Monitoring, Protection and Remediation pp. 403-416.
- Nuzhat S, Ashok KP, Azra NK, Basharat M (2015) Heavy metal accumulation by Azolla pinnata of dal lake ecosystem, India. Journal of Environment Protection and Sustainable Development 1(1): 8-12.
- Punita SP, Soma KM (2015) Capacity of Azolla pinnata var. imbricata to absorb heavy metals and fluorides from the wastewater of oil and petroleum refining industry at Vadodara. IJAPRR 2(1): 37-43.
- Ranjana JT, Jeya R, Prabha JMP (2012) phytoaccumulation of chromium and copper by Pistia stratiotes and Salvinia natans (L.) all. Plant Resour 2(6): 725-730.
- Santosh KP, Neelima M, Shivangee S (2012) Phytoremediation of chromium and cobalt using Pistia stratiotes: A sustainable approach. Proceedings of the International Academy of Ecology and Environmental Sciences 2(2): 136-138.
- Uysal Y (2013) Removal of chromium ions from wastewater by duckweed, Lemna minor by using a pilot system with continuous flow. Journal of Hazardous Materials 263(2): 486-492.
- Wang Q, Cui Y, Dong Y (2002) Phytoremediation of polluted waters: Potentials and prospects of wetland plants. Engineering in Life Sciences 1(2): 199-208.
- Xiaomei L, Maleeya K, Prayad P, Kunaporn H (2004) Removal of cadmium and zinc by water hyacinth, Eichhornia crassipes. Science Asia 30: 93-103.
© 2025 © K Usharani. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






