- Submissions

Full Text
COJ Nursing & Healthcare
Research Progress of Non-Coding RNA in Chronic Rhinosinusitis
Shun Ding, Qichao Hong, Jinren Yan and Zhonglin Mu*
Department of Otolaryngology Head and Neck Surgery, China
*Corresponding author: Zhonglin Mu, Department of Otolaryngology, Head and Neck Surgery, The First Affiliated Hospital, Haikou, China
Submission: March 11, 2022;Published: March 21, 2022

ISSN: 2577-2007Volume7 Issue5
Abstract
Chronic Rhinosinusitis (CRS) is one of the common diseases of the upper respiratory tract, with a high incidence and huge economic loss. Despite receiving the best medical and surgical treatment, some patients have persistent or recurrent symptoms. The pathogenesis mechanism of CRS is not clear. Studies have shown that Non-Coding RNA (ncRNA) plays an important role in the occurrence and development of CRS. Clarifying the mechanism of the occurrence and development of ncRNA in CRS is conducive to enriching the mechanism of CRS and providing a scientific basis for its treatment. This paper mainly reviewed the role of ncRNA in the pathogenesis of CRS.
Keywords: Chronic rhinosinusitis; Non-coding RNA; Pathogenesis
Introduction
CRS is an inflammatory disease involving the mucous membranes of the sinuses and nasal cavity that often lasts more than 12 weeks. The clinical phenotype of CRS is often distinguished by the presence or absence of nasal polyps and, depending on the type, 2020 EPOS classifies CRS as Type 2 CRS and non-Type 2 CRS [1]. Clinical manifestations include nasal congestion, nasal leakage, stuffiness or pain in the head and face, and a decreased or lost sense of smell [1,2]. The pathogenesis of CRS is still unclear, and Lam K et al. [3] proposed six main hypothesis mechanisms, including biofilm, microbial, fungal, superantigen, arachidonic hypothesis, and immune barrier.
However, these hypotheses cannot fully elucidate the pathogenesis of CRS [3]. In recent years, genetic regulation has also emerged as a key factor in the pathogenesis of CRS. Non-coding RNAs are a class of RNAs that are not involved in coding for proteins, but this does not mean that they do not contain genetic information and are not functional. It has recently been shown that most genomes of mammals and other complex organisms are transcribed into ncRNAs, many of which are spliced or processed into smaller products. These ncRNAs include microRNA (miRNA), small interfering RNA (siRNA), long non-coding RNA (lncRNA), small nucleolar RNA (snoRNA), other small regulatory RNAs, longer transcripts (including complex interlaced patterns), and overlapping sense and antisense transcripts, most of which are of unknown function. The primary function of these RNAs is to transduce signals that control the expression of various genes in physiology and development, such as chromatin structure and epigenetic memory, transcription, RNA splicing, editing, translation, and updating [4]. Studies have shown that lncRNA, miRNA, and siRNA are all involved in the development of CRS, and new drugs for CRS have been developed around their mechanisms of action. Therefore, this paper summarises the regulatory mechanisms of CRS from these three aspects and provides a theoretical basis for the study of therapeutic approaches to CRS.
Material and Method
MiRNAs are a class of tissue-specific ncRNAs, approximately 22 nucleotides long. miRNAs regulate gene expression primarily through inhibition of translation or mRNA degradation, maintain cellular homeostasis through negative gene regulation, and are highly conserved genetically [5]. A balanced physiological environment requires control of the correct expression of miRNAs, as these small molecules are involved in the regulation of various genetic pathways, from cell cycle checkpoints and cell proliferation to apoptosis [6]. MiRNAs are also involved in various aspects of the development of CRS, which are described next in terms of Epithelial-Mesenchymal Transition (EMT), eosinophil transport, and inflammatory response.
MiRNA21 regulates EMT in CRS through the TGF-β1 signaling pathway
EMT is a fundamental biological process that involves normal physiological processes such as embryogenesis, wound healing, and tissue repair, as well as many pathological processes, including organ fibrosis, malignant transformation, and cancer progression [7]. It has been demonstrated that EMT features are prevalent in CRS sinus and nasal polyp tissue [8] and that Transforming Growth Factor (TGF-1), a representative cytokine in tissue repair and remodeling, initiates tissue remodeling in airway epithelium and nasal tissue by activating EMT signaling [9]. TGF-β1 plays an essential role in the formation of nasal polyps, the promotion of mucosal remodeling, the stimulation of fibrosis (by attracting stromal cells), angiogenesis, and the accumulation of extracellular matrix [10]. Li X et al. [11] found a significant increase in miR-21 expression in CRSwNP.
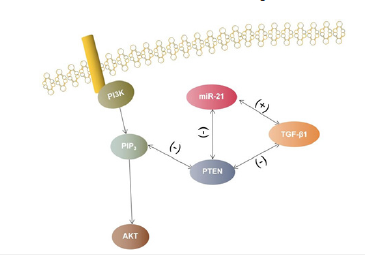
miR-21 may promote TGF-β1-mediated EMT in Human Nasal Epithelial Cells (HNEC) in response to the PTEN/Akt signaling pathway. Cellular experiments confirmed that TGF-β1 treatment in HNEC caused miR-21 upregulation, while expression of miR- 21 alone also resulted in EMT-like transformation in HNEC. In HNEC, EMT was also significantly inhibited by down-regulation of miR-21. In parallel, phosphatase and tensin homolog (PTEN) is thought to be an inhibitor of the PI3K/AKT pathway, and the PI3K/ AKT pathway is central to EMT regulation [12]. To confirm the relationship between miR-21 and PTEN in the EMT of CRSwNP, the study used miR-21 targeting molecular techniques and identified PTEN, Akt, and p-Akt as miR-21 target genes by online prediction. PTEN, Akt, p-Akt, and TGF-β1 treatment were measured in miR-21- mediated TGF-β1-induced EMT of HNECs, resulting in decreased PTEN and increased Akt phosphorylation, which was counteracted by downregulation of miR-21. In addition, overexpression of miR-21 alone in HNECs downregulated PTEN and increased Akt phosphorylation, whereas Akt inhibitor III, a specific inhibitor for Akt activation, significantly reduced miR-21-regulated EMT [11]. The above pathways can be attributed to the miR-21-TGF-β1- PTEN-AKT pathway, and the mechanism of action of this pathway is shown in Figure 1.
Figure 1:Diagram of the miR-21-TGF-β1-PTEN-AKT pathway.
Endothelial miRNA-1 regulates eosinophil transport in CRS
Eosinophilic Cells (EOS) are the main pathological feature of CRS, especially in the CRSwNP where infiltration is significantly increased [13-15]. Persistent eosinophilic inflammation refers to the prolonged survival of eosinophils and their prolonged accumulation in the tissues [16-18]. In patients with eosinophilic sinusitis, EOS accumulates in the superficial lamina propria and the cytoplasm is rich in cytotoxic particles that release several types of cytokines, chemokines, and lipid mediators
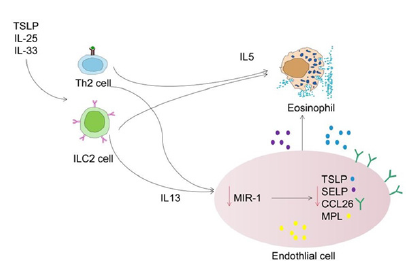
which contribute to increased inflammation and clearance of pathogens [19]. The clinical relevance of miRNA-1 revealed a negative correlation between miR-1 levels and EOS in patients with CRS. Furthermore, stimulation of the Th2 cytokine IL-13 resulted in a dose-dependent decrease in miR-1 in human lung tissue and isolated endothelial cell cultures. These suggest that low miR-1 levels play a key role in the Th2-mediated EOS response. Selective overexpression of miR-1 in endothelial cells suppresses airway eosinophilia, airway hyperresponsiveness, and macrocytosis. Mechanistically, miR-1 recruits multiple eosinophil genes to the RNA-induced Silencing Complex (RISC) and directly inhibits the IL-13-induced binding of eosinophils to the endothelial surface. Based on these findings, miR-1 targets were negatively correlated with miR-1 and directly correlated with eosinophils in tissues. This suggests that miR-1 controls the increase of EOS in tissues by regulating the network of transporter genes in the endothelium [20] and promotes the progression of CRS. The mechanism of miR- 1 action on Type 2 CRS is shown in Figure 2.
Figure 2:Mechanism of action of miR-1 in Type 2 inflammation.
MiR124-mediated expression of AHR regulates the inflammatory response to CRS induced by nasal polyps
MiRNAs are a component of the innate immune response and can inhibit inflammatory signaling. MiR124 is an important member of the miRNA family associated with inflammation, and aberrant miR124 expression has been observed in many inflammatory and immune diseases. The Aryl Hydrocarbon Receptor (AHR) is a cytoplasmic receptor originally identified for its key role in mitigating the toxicity of environmental pollutants. However, recent evidence suggests that the AHR responds to exogenous and endogenous signals from multiple sources, including diet, host metabolism, and gut microbes [21-23]. When bound to a ligand, the functional AHR acts as a transcription factor via the promoter to influence the binding of gene expression and is involved in the recruitment of coactivators and co-blockers to specific DNA regions.
At the same time, AHR is essential for broad immune function, controlling inflammation in feedback loops whether at a steadystate or an ongoing inflammatory response, and also maintaining the function of innate and adaptive cell populations at mucosal barrier sites [24]. MiR124 was found to play an important role in the inflammatory response to CRS caused by nasal polyps. MiR124 expression was reduced in nasal polyps and negatively correlated with AHR expression [25]. MiR124 can directly target the 3’ untranslated region (3’-UTR) of AHR, thereby inhibiting AHR expression. To further explore the relationship between miR124, AHR, and CRS inflammatory response, HNEC cells were transfected with miR124 mimics, miR124 inhibitors, or siRNAs of AHR, and the results showed that miR124 could enhance the inflammatory response of cells by negatively regulating the expression of AHR. Thus, the regulation of AHR expression by miR124 is critical for the development of inflammatory responses in CRSwNPs.
lncRNA XLOC_010280 Mediates CCL18 to Regulate the CRS Inflammatory Response
lncRNA is an RNA molecule with a specific function, with at least 200 bases, and without the ability of RNA to encode proteins [26]. LncRNA was originally thought to be a non-functional by-product of RNA polymerase II transcription products but was also disputed to be “pseudo-transcriptional noise” [27]. However, experimental studies have shown that lncRNAs play an essential role in cell development and metabolism, including cell cycle regulation, transcription, splicing, genetic imprinting, gene rearrangement, chromatin modification, etc [28,29]. Little research has been done on the role of LncRNA in CRS, and some experiments have explored the role of LncRNA in CRS.
The lung activation regulatory chemokine (CCL18) is a 7.8kDa protein consisting of 69 amino acids. Peterson et al. [30]. found that CCL18 mRNA was significantly increased in NP (P < 0.001) and UT (P < 0.05) of CRSwNP patients compared to Unfused Tissue (UT) of normal controls, whereas CCL18 mRNA was not increased in UT of CRSsNP patients. Therefore, the overexpression of CCL18 may be related to the pathogenesis of CRSwNP [31]. CCL18 was upregulated in CRSwNP patients, as was the adjacent lncRNA XLOC_010280. Exploring the potential function of dysregulated CRSwNP lncRNAs and the target protein-coding genes regulated in trans by cis revealed that lncRNAs have cis-regulatory effects on neighboring protein-coding genes. The study by Wang et al. [32] identified the important lncRNA XLOC_010280, which is highly associated with cis-CCL18. CCL18 was found to be enriched in predicted lncRNA function and XLOC_010280 was identified as an adjacent lncRNA to CCL18. Both CCL18 and XLOC_010280 were upregulated in CRSwNP patients. Thus, lncRNA XLOC_010280 upregulates mRNA expression of CCL18, and both XLOC_010280 and CCL18 are located in the chromosome 17q11.2-q12 CC chemokine gene cluster, which also includes CCL3, CCL4, CCL5, and CCL23 and is closely associated with eosinophilic inflammation [33]. These XLOC_010280 plays a major role in eosinophilic inflammation by regulating the expression level of CCL18.
SiRNA interference with VEGF expression in CRS
SiRNA is a powerful tool for regulating gene expression through RNA silencing. Double-stranded RNA oligonucleotides induce cleavage of homologous target transcripts, resulting in posttranscriptional silencing of potentially any gene. In this way, siRNAs can be used as novel drugs for a wide range of diseases and have become a hot topic in the development of CRS-targeted drugs [34].
Vascular Endothelial Growth Factor (VEGF) promotes the growth of nasal epithelial cells, leading to the proliferation of sinus mucosa. Therefore, downregulation of VEGF may inhibit mucosal proliferation. Cao C et al. [35] found that the use of siRNAs targeting VEGF silenced VEGF expression and prepared injectable deacetylated chitosan hydrogels suitable for sinus injection and with long-term retention capacity as siRNA carriers. Human bronchial epithelial cells were cultured directly on the hydrogel to observe biological behavior in vitro, or the hydrogel could be injected into the sinus cavity for in vivo studies [35]. Following the introduction of siRNA, VEGF expression was significantly suppressed in bronchial epithelial cells at the mRNA and protein levels. The number of viable cells on the gel was significantly reduced, resulting in an inhibition of proliferation. However, the cytoskeletal arrangement of the remaining cells was largely unaffected. Hydrogels were able to retain siRNA for longer periods, allowing for the sustained release of siRNA. In vivo sinus, the mucosal analysis showed that siRNA was able to bind to cells and that mucosal thickness was significantly reduced. It was concluded that chitosan-based injectable hydrogels targeting VEGF treated with siRNA could be a convenient treatment option for CRS.
Summary and Prospects
With the development of scientific research technology, ncRNAs play an important role in the diagnosis and treatment of CRS, but their research into the pathogenesis of CRS is still in the exploration stage. With the improvement of detection technology, in the future, apart from ncRNA, the role of the CRS-related genome, methylation of genes and exosomes will also be discovered one after another, providing a cutting-edge theoretical basis for the development of safe, fast, and efficient targeted drugs and therapeutic measures for CRS.
Authors’ Contributions
Study design: ZLM. Data acquisition: SD, QCH. Data analysis: SD, QCH. Data interpretation: SD, QCH, JRY. Drafting of the manuscript: SD, QCH. Revision of the manuscript: SD,QCH, JRY, All authors read and approved the final manuscript.
References
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, et al. (2020) European position paper on rhinosinusitis and nasal polyps. Rhinology 58: 1-464.
- Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, et al. (2017) Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy 72(2): 274-281.
- Lam K, Schleimer R, Kern RC (2015) The etiology and pathogenesis of chronic rhinosinusitis: A review of current hypotheses. Current Allergy and Asthma Reports 15(7): 1-10.
- Mattick JS, Makunin IV (2006) Non-coding RNA. Human Molecular Genetics 15(Suppl1): R17-R29.
- Correia de Sousa M, Gjorgjieva M, Dolicka D, Cyril S, Michelangelo F, et al. (2019) Deciphering miRNAs’ action through miRNA editing. International Journal of Molecular Sciences 20(24): 6249.
- Mishra S, Yadav T, Rani V (2016) Exploring miRNA based approaches in cancer diagnostics and therapeutics. Critical Reviews in Oncology/Hematology 98: 12-23.
- Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer 2(6): 442-454.
- Lee HM, Kang JH, Shin JM, Seoung AL, Il-Ho P (2017) Chemical chaperone of endoplasmic reticulum stress inhibits epithelial-mesenchymal transition induced by TGF-β1 in airway epithelium via the c-src pathway. Mediators of Inflammation 2017: 8123281.
- Park IH, Kang JH, Shin JM, Heung ML (2016) Trichostatin A inhibits epithelial mesenchymal transition induced by TGF-β1 in Airway Epithelium. PloS one 11(8): e0162058.
- Balsalobre L, Pezato R, Perez NC, Maria TSA, Rodrigo PS, et al. (2013) Epithelium and stroma from nasal polyp mucosa exhibits inverse expression of TGF-β 1 as compared with healthy nasal mucosa. Journal of Otolaryngology-Head & Neck Surgery 42(1): 1-5.
- Li X, Li C, Zhu G, Yuan W, Xiao Z, et al. (2019) TGF-β1 induces epithelial-mesenchymal transition of chronic sinusitis with nasal polyps through microRNA-21. International Archives of Allergy and Immunology 179(4): 304-319.
- Thiery JP (2003) Epithelial-mesenchymal transitions in development and pathologies. Current Opinion in Cell Biology 15(6): 740-746.
- Akdis CA, Bachert C, Cingi C, Mark SD, Peter WH, et al. (2013) Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. Journal of Allergy and Clinical Immunology 131(6): 1479-1490.
- Payne SC, Borish L, Steinke JW (2011) Genetics and phenotyping in chronic sinusitis. Journal of Allergy and Clinical Immunology 128(4): 710-720.
- Lee S, Lane AP (2011) Chronic rhinosinusitis as a multifactorial inflammatory disorder. Current Infectious Disease Reports 13(2): 159-168.
- Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, et al. (1997) Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. The Journal of Immunology 158(8): 3902-3908.
- Kowalski ML, Grzegorczyk J, Pawliczak R, et al. (2002) Decreased apoptosis and distinct profile of infiltrating cells in the nasal polyps of patients with aspirin hypersensitivity. Allergy 57(6): 493-500.
- Fan GK, Itoh T, Imanaka M, Takenaka H (2000) Eosinophilic apoptosis in sinus mucosa: Relationship to tissue eosinophilia and its resolution in allergic sinusitis. Journal of allergy and clinical immunology 106(3): 551-558.
- Miyata J, Fukunaga K, Kawashima Y, Takashi W, Akina S, et al. (2019) Dysregulated fatty acid metabolism in nasal polyp‐derived eosinophils from patients with chronic rhinosinusitis. Allergy 74(6): 1113-1124.
- Korde A, Ahangari F, Haslip M, et al. (2020) An endothelial microRNA-1-regulated network controls eosinophil trafficking in asthma and chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 145(2): 550-562.
- Yamada T, Horimoto H, Kameyama T, Sumio H, Hiroaki Y, et al. (2016) Constitutive Aryl Hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nature immunology 17(6): 687-694.
- Rothhammer V, Mascanfroni ID, Bunse L, Maisa CT, Jessica EK, et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the Aryl Hydrocarbon receptor. Nature Medicine 22(6): 586-597.
- Rothhammer V, Borucki DM, Tjon EC, Maisa CT, Chun CC, et al. (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557(7707): 724-728.
- Shinde R, McGaha TL (2018) The Aryl Hydrocarbon receptor: Connecting immunity to the microenvironment. Trends in Immunology 39(12): 1005-1020.
- Liu CC, Xia M, Zhang YJ, Jin P, Zhao L, et al. (2018) Micro124-mediated AHR expression regulates the inflammatory response of chronic rhinosinusitis (CRS) with nasal polyps. Biochemical and Biophysical Research Communications 500(2): 145-151.
- Spizzo R, Almeida MI, Colombatti A, Calin GA, et al. (2012) Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene 31(43): 4577-4587.
- Struhl K (2007) Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nature Structural & Molecular Biology 14(2): 103-105.
- Hung T, Wang Y, Lin MF, Ashley KK, Yojiro K, et al. (2011) Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nature Genetics 43(7): 621-629.
- Huarte M, Guttman M, Feldser D, Manuel G, Magdalena JK, et al. (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142(3): 409-419.
- Peterson S, Poposki JA, Nagarkar DR, Regina TC, Anju TP, et al. (2012) Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 129(1): 119-127.
- Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, et al. (1997) A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. The Journal of Immunology 159(3): 1140-1149.
- Wang W, Gao Z, Wang H, Taisheng Li, Wei He, et al. (2016) Transcriptome analysis reveals distinct gene expression profiles in Eosinophilic and Noneosinophilic Chronic Rhinosinusitis with nasal polyps. Scientific Reports 6(1): 1-14.
- Guttman M, Donaghey J, Carey BW, Manuel G, Jennifer KG, et al. (2011) lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477(7364): 295-300.
- Roberts TC, Ezzat K, Andaloussi SEL, Marc SW, et al. (2016) Synthetic SiRNA delivery: Progress and prospects. SiRNA Delivery Methods 1364: 291-310.
- Cao C, Yan C, Hu Z, Shao Zhou (2015) Potential application of injectable chitosan hydrogel treated with siRNA in Chronic Rhinosinusitis therapy. Molecular Medicine Reports 12(5): 6688-6694.
© 2022 Zhonglin Mu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






