- Submissions

Full Text
Aspects in Mining & Mineral Science
Critical Factors for Effective Anti-Corrosion Coatings
Elif Suna Koçyiğit*
Kanat Paints and Coatings, Kemalpaşa Industrial Zone, Kemalpaşa, İzmir, Turkey
*Corresponding author:Elif Suna Koçyiğit, Kanat Paints and Coatings, Kemalpaşa Industrial Zone, Kemalpaşa, İzmir, Turkey
Submission: March 19, 2025: Published: March 28, 2025

ISSN 2578-0255Volume13 Issue 3
Opinion
Anticorrosive coatings are inevitable when metals are in question whole over the world considering lifespan of materials, safety and economic effects. When steel surfaces are exposed to oxygen and moisture, an oxidation/reduction reaction takes place, and the product of this reaction is iron oxide, commonly known as rust. To inhibit these electrochemical process, organic coatings are in the foreground which provide a barrier to the corrosive elements of nature, and delay the propagation of rust. The options for anti-corrosion coatings are broad but they can be simply put into three main groups; barrier, inhibitive and sacrificial. The more stringent environmental standards and sustainability concerns are pushing forward water-based coatings for corrosion protection. Solvent based systems accounted for more than half of the global anti-corrosion coating market share in 2023, on the other hand the growth rate for water-based coating technology is higher than that for conventional solvent-based coatings [1].
To achieve required corrosion performance to the paint, major concerns is proper adhesion to the substrate [2]. The possible interactions between paint and substrate are covalent bonds, mechanical interlocking, polar interactions (acid base interactions) and hydrogen bonding or salt-complex formation [3]. However, all these bonds are sensitive to water. Essentially, chemical and mechanical adhesion are two components of that contribute to overall adhesion of a coating. Chemical adhesion arises from chemical bonding between the coating and the substrate by way of wettability of a surface by the paint. Covalent chemical bonds are the foremost bonding which play a crucial role on degree of adhesion. Metallic and ionic bonds are not such important bonding types for organic coatings on metals. Mechanical adhesion related to the surface roughness by contact of metal and coating to generate a mechanical interlock. It is obvious that increasing the surface roughness improves the adhesion. In general terms, the greater the (wet) adhesion strength, the better the performance. Even so, there are also opposing views that defend the effect of wet adhesion on anti-corrosion performance is little. The polymeric binder is also important and is assumed to contribute to adhesion of the coating to the substrate. In some cases, hybrid or blend of different binders can be used in same paint according to the application and performance requirements [4-6].
Another important issue is to achieve barrier protection to achieve corrosion protection. Basically, if moisture cannot contact the substrate, aqueous corrosion cannot occur. This is related to permeability, diffusion and solubility coefficients for polymers found in organic resins in paint. All organic coatings exhibit different levels of solubility and permeability against the components of the corrosive environment, which can be described as ionic conductivity. For example, diffusion coefficients for oxygen in many polymers are within an order of magnitude of the diffusion coefficient of oxygen dissolved in water [5,6]. Water diffusion in polymers is a hot topic since the water is a transmission medium of oxygen and ions and delamination of organic polymers occurs when water diffused through the metal substrate. As thermodynamically, stronger hydrogen bonds are easily formed between the water and oxygen functional groups in polymers, which may destroy the weaker between polymers and metal and hence delamination occurs. Therefore, as long as water reached the interface, polymer delamination would occur due to the destruction polymers and metal bonding. But there is an opposing view that defend the influence of water was reversible, meaning that when water left to form a stronger hydrogen bond, the bond between polymers and metal turned to its original state. Even though coatings are often considered to be homogeneous materials at the macroscale, their molecular structures are strongly heterogeneous, due to the polymer chemistry, geometry and the crosslinking reactions taking place. This is also having an influence on diffusion pathway of water. It can be evincible that water reaching the metal/polymer interface is sufficient for polymer delamination to begin and the schematic illustration of corrosion is shown in Figure 1.
Figure 1:Schematic illustration of corrosion.
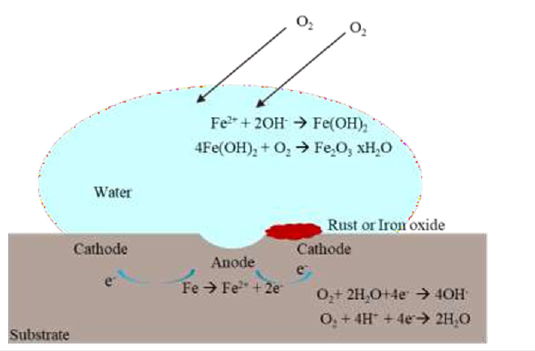
These reactions result in an alkaline pH at the interface, ending up a reduction in wet adhesion strength and subsequent delamination [3,7]. It can be clearly stated that there is no single acceptable theory that explain all the possibility of water-polymer interactions. One more issue that needs to be considered when talking about anticorrosion coatings is the curing conditions. Mechanical strength, corrosion resistance and adhesion strength of coatings are thoroughly influenced by the curing temperature. The degree of cross-linking between the polymer chains are related to the curing temperature, which further has an impact on the structural integrity of the coating. Sufficient crosslinking creates an immobilized structure in the polymer-substrate bonding region that delays cathodic delamination and improves adhesion. This process is also strongly depended on the coating thickness which is an essential parameter for assuring barrier protection against corrosion [3,8,9]. From one point of view, increasing the thickness of the paint film decreases its permeability because increasing the diffusion pathway for water and ions.
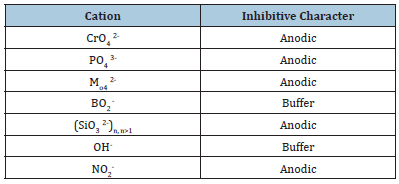
A good painted film that is a must for anticorrosive coatings should exhibit a perfect combination of “hardness and plasticity”. The films mechanical properties also depend on the presence of defects or heterogeneities, the internal stresses and the Glass transition temperature (Tg). Tg is a vital parameter that must be worth- stressing separately. The role of the Tg is also important, because, at temperatures below Tg, the crystallised polymer shows increased hardness, whereas deformation improves in temperatures higher than Tg. This is because the mechanical properties such as elastic modulus of polymer materials are changed remarkably above and below the Tg. Polymers with a higher Tg tend to have higher tensile strength, as the increased molecular mobility at elevated temperatures allows for better alignment and bonding. Also, polymers with a higher Tg typically exhibit a higher elastic modulus, indicating greater stiffness and rigidity. So, as the Tg increases, the polymer becomes more brittle and prone to fracture upon impact. Inversely, polymers with lower Tg has better impact resistance and toughness [3]. Anticorrosive pigments must be included in anticorrosive coating formulations. Conventional corrosion inhibiting pigments are generally metal or metal oxide powders. Electrochemically active metals, mainly Zn, are added to organic resins to ensure sacrificial cathodic protection. Salts of chromate such as strontium chromate, are also effective corrosion inhibitors but sadly, chromate is carcinogenic, toxic and environmentally hazardous. Therefore, introducing benign and environmentally friendly active inhibitors is in vital. There are number of chromate-free pigments with anticorrosive properties such as phosphate, molybdate, vanadate, silicate, borate and lithium. Inhibitive character of various inhibitors are shown in Table 1, [10]. Promising potential alternatives for chromate containing pigments include phosphate containing pigments. Zinc phosphate is the first generation of phosphate containing pigments. However, because of the low solubility of zinc phosphate, corrosion inhibition is relatively poor. Its solubility and phosphate content will be increased by physical and chemical modifications. Pigment solubility is also crucial in terms of corrosion because too low a solubility leads to incomplete corrosion protection but too high a solubility creates osmotic blistering. By the way multiple generations of phosphate containing pigments such as zinc aluminium phosphate, zinc aluminium polyphosphate and strontium aluminium polyphosphate can be developed. Physically, when an electrolyte permeates the coating, the pigment dissolve and releasing anions and cations which slow the anodic or cathodic side reactions. Some corrosion inhibitors also change solution pH to medium site. For example, steel passivation is promoted under alkaline medium and hence pigments that change the pH to 8 or greater are preferred. The pigment-binder interaction is also very important to obtain an effective barrier protection. Large pigment agglomerates must be avoided to inhibit defects in the coating to get optimal anticorrosion performance. Improving the pigmentbinder interactions enhances the barrier properties of the coating and effectively increases the volume occupied by the pigment/filler [1,6]. Nowadays, the use of sustainable polymers in anticorrosive coatings has been of considerable attention. Moreover, self- healing anti-corrosion coatings are a new type of intelligent materials that can autonomously repair themselves after experiencing mechanical damage.
Table 1:Schematic illustration of corrosion.
To summarize all these issues, anti-corrosion coatings effectively protect metal surfaces from degradation when the necessary conditions are met. The latest advancements in anti-corrosion technology push market to low VOC, smart and nanotechnologybased coatings to meet sustainability requirements.
References
- Elizalde O, Amthor S, Moore C (2010) Water and solventborne anticorrosion coatings via new binder concepts. JCT Coatings Tech 7(9): 22-31.
- Kutz M (2018) Handbook of environmental degradation of materials. William Andrew, pp. 367-385.
- Kefallinou Z (2017) The impact of curing time on the electrochemical behaviour of intact epoxy-phenolic coatings on tinplate and tin-free steel. The University of Manchester, United Kingdom.
- Lyon SB, Bingham R, Mills DJ (2017) Advances in corrosion protection by organic coatings: What we know and what we would like to know. Progress in Organic Coatings 102: 2-7.
- Kandeloos AJ, Attar MM (2023) The diffusion and adhesion relationship between free films and epoxy coated mild steel. Progress in Organic Coatings 179: 107561.
- Buchheit RG (2005) Corrosion resistant coatings and paints. Handbook of Environmental Degradation of Materials. William Andrew Publishing, pp. 367-385.
- Yang C, Xing X, Li Z, Zhang S (2020) A comprehensive review on water diffusion in polymers focusing on the polymer-metal interface combination. Polymers 12(1): 138.
- Khan AA, Khan A, Zafar Z, Ahmad I (2023) Investigating the effect of curing temperature on the corrosion resistance of epoxy-based composite coatings for aluminium alloy 7075 in artificial seawater. RSC Advances 13(30): 21008-21020.
- Otmačić Ćurković H, Kapitanović A, Filipović M, Gorišek P (2024) The effect of coating drying conditions on bronze corrosion protection. Journal of Electrochemical Science and Engineering 14(2): 247-263.
- Sørensen PA, Kiil S, Dam-Johansen K, Weinell CE (2009) Anticorrosive coatings: A review. Journal of Coatings Technology and Research 6: 135-176.
© 2025 Elif Suna Koçyiğit. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






