- Submissions

Full Text
Research in Medical & Engineering Sciences
Acute Gastroenterıtıs Agents Under 5 Years Old Age Chıldren
Çiğdem Eda Balkan1* and Demet Çelebi2
1Department Microbiology, Kafkas University, Turkey
2Department Microbiology, Atatürk University, Turkey
*Corresponding author: Çiğdem Eda Balkan, Department Microbiology, Kafkas University , Turkey
Submission: August 31, 2017; Published: November 13, 2017

ISSN : 2576-8816Volume2 Issue2
Abstract
Aim: Acute gastroenteritis outbreaks common health problem throughout the world especially in children. Everyyear thousands of children dies because of the diarrhea cause of bacterias, parasites and viral diseases.In this study we aim to find the rates of the agents cause diarrhoea, children under 5 years old according to the seasons.
Matherials and methods: In this study 216 stool samples ,children under 5 years old age ,are examine with some tests for Rotaviruses, Adenoviruses, Salmonella, Shigella, Entemoeba and Giardia, Clostridium difficile(ToxinA, Toxin B). Gastroenteritis were tested for Clostridium difficile by means of enzyme-linked immunoassay(ToxinA, Toxin B). CerTest Rotavirus and Adenovirus Card Test (CerTest, Biotec, Spain), a qualitative immunochromatographic assay was used to detect rotavirus and adenovirus antigens.This immunochromatic tests used for detecting Giardia and Entemoeba antigens too and most of the cases we support our results with the microscopy. They were also examined by ELISA for Clostridium difficile cytotoxins A and B. We used macconcey medium for finding the lactose negative colonies after that selenit-f media used for detecting only salmonella and shigella in the stool samples, the final results has been reached by the IMVIC tests.
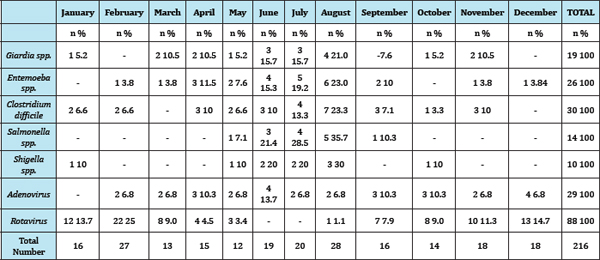
Result: Infections results 40.74% rotavirus (adenovirus 13.42%, 5 case is mixed infections ) , bacteria 24.98% and parasites 20.82% . Some agents are peak in the cold seasons for example most Rotavirus outbreaks increased in winter.(November 11.36%, December 14.77%, January 13.76%, February 25% ). In contrast some outbreaks occured in hot seasons especially Salmonella ( June 21.42%, July 28.57%, August 35.71%) and Shigella (June 20 %, July 20% ,August 30%)(Table 1).
Conclusion: This study find the highest proportion of dual diarhoeal infections was identified in February, March and April and seasonal occurrence of some mono-infections; infection by rotavirus is more frequent in winter and spring In conclusion we believe that analysis of viral antigens,bacterias and the parasites as a diarrheal agents in stool sample is important in 0-5 years old infants because of hospitalizations and unneccessary drugs.
Keywords: Child; Diarrhea; Gastroenteritis
Introduction
Acute gastroenteritis is one of the most common health problems worldwide [1]. Acute infectious diarrhea is a common disease in young children throughout the world too. Estimated incidence rates in developing countries range from 3.5 to 7.0 episodes per child per year during the first 2 years of life and from 2 to 5 episodes per child per year for the first 5 years [2]. Pediatric diarrhea is a costly disease in terms of direct (and indirect) monetary costs to each community, and it is a cause of emotional trauma for the child and the parents [3].
More than 700 million cases are estimated to occur annually in children less than 5 years of age, resulting in few deaths in developed countries, but more than 2 million deaths in developing countries. Worldwide, a diverse group of viral, bacterial, and parasitic pathogens cause acute enteric symptoms including nausea, vomiting, abdominal pain, fever, and acute diarrhea. Infections with viral agents, unlike those with bacterial or parasitic pathogens, cannot be treated with antibiotics, and many cannot be prevented by improvements in quality of drinking water, food, or sanitation. Until the early 1970s most viral agents causing gastroenteritis in humans were largely unknown. Studies using electron microscopy of intestinal contents resulted in the discovery of numerous viral enteropathogens now classified Rotaviruses, 'enteric' Adenoviruses or other viruses which can cause gastroenteritis [1].
Among them, viral infection is the most common cause, followed by bacterial and parasitic infections [4]. Giardia lamblia, Entamoeba histolytica, are the major parasitic agents [5]. Salmonella spp and Shigella spp remain among the bacteria most frequently isolated from stool samples obtained from diarrhea patients, especially in rural areas from developing countries [6,7]. Other of this diarrheal agents Clostridium difficile is emerging as a major cause of childhood diarrhea in both community and hospital settings [8,9]. Clostridium difficile infection has more recently been implicated as an increasingly prevalent diarrheal pathogen in children [10-12]. Moreover, evidence suggests that a large proportion of pediatric Clostridium difficile cases are community-acquired infections and that many of these infections lack the traditional risk factor of exposure to antimicrobial drugs [13-15]. In this study we aim to find the prevelance of these gastroenteritis agents under 5 years old children .
Materials and Methods
The study group comprised 216 children admitted consecutively for diarrhea to this hospital during a period of one year. The patients were chosen if they had diarrhoea on admission, irrespective of other concomitant diseases. Children who had been treated with antibiotics before the onset of diarrhoea were included too. Samples were obtained by direct deposition in a sterile container and were transported the same day to hospital laboratories, where they were stored at 4-8 °C until they were processed. Specimens for bacteriological culture were inoculated into appropriate media on the day of collection.
Stool specimens from each infant with severe gastroenteritis were tested for Clostridium difficile by means of enzyme-linked immunoassay. CerTest Rotavirus and Adenovirus Card Test (CerTest, Biotec, Spain), a qualitative immunochromatographic assay was used to detect Rotavirus and Adenovirus antigens.This immunochromatic tests used for detecting Giardia and Entemoeba antigens too and most of the cases we support our finding with the microscopy. They were also examined by ELISA for Clostridium difficile cytotoxins A and B. We used Macconcey medium for detecting the lactose negative colonies after that Selenit-F media used for detecting only Salmonella and Shigella in the stool samples. We don't find the serotypes of this bacterias. In this study we use IMVIC tests define as symptoms of diarrhoea not attributable to another cause.
Result
The total number of children admitted during the 1 year of survey was 216 children. Rotaviruses were the single most common pathogen. For all agents peak incidence occured in February (n=27) and August (n=28) (Table 1). Adenoviruses present in 29 of the total number of 216(13.42%) patients. Clostridium difficile strains isolated from 30 of 216(13.88%) ,parasites including ; Giardia and Entemoeba were identified in 19(8.79%) and 26(12.03%)(Table 1).
Some agents are peak in the cold seasons for example most Rotavirus outbreaks increased in winter .(December 14.77%, January 13.76%, February 25%) Looking at all the months, concluded that there isn't significant difference about Adenovirus infections.We found 5 Adenovirus-Rotavirus mix infections 2 case in February , 1 case in March and 3 case in April (Table 1). In contrast some outbreaks occured in hot seasons especially Salmonella (June 21.42%, July 28.57%, August 35.71%) and Shigella (June 20%, July 20%, August 30%) (Table 1).
Table 1: Number of gastroenteritis agents.
Discussion
Acute gastroenteritis in children continues to be a significant problem throughout the world.Worldwide more than 744-1000 million cases of acute diarrhoeal disease are estimated to ocur annually just in children under 5 years old [16]. Of the estimated total 10.6 million deaths among children under five years of age worldwide, 42 percent occur in the World Health Organization (WHO African region) [17]. Throughout the world global estimates of the number of deaths due to diarrhea have shown like [18], from 4.6 million in the 1980s [16] to 3.3 million in the 1990s [19] to 2.5 million in the year 2000 [20].
Some studies refers that enterotoxigenic Rotaviruses predominate in developing areas, cytotoxigenic Clostridium difficile are seen with increasing frequency in developed areas; and Shigella, Salmonella, and Giardia lamblia are found throughout the world [21]. In Netherland viruses were detected in 82% of the samples, with Rotavirus being most common (56%), bacteria in 32% and parasites in 10% [22]. Our study consistent with these study 40.74% Rotavirus (Adenovirus 13.42% 5 case is mixed infections), bacteria 24.98% and parasites 20.82% (Table 1). Most of the studies mention that mixed infections are less frequent than mono-infections , but the rate of double infections varies widely in the literature [23]. A study in Spain find the most frequent mixed infections were Rotavirus-Astrovirus (13 cases) and RotavirusAdenovirus (10 cases in 820 stool samples) [23] In this study Rotavirus and Adenovirus mix enfection 5 of 216 sample (2.31%) (Table 1). This study find the highest proportion of dual infections was identified in February,March and April and seasonal occurrence of some mono-infections; infection by Rotavirus is more frequent in winter and spring . In Spain most of the cases of mixed infection occurred in autumn (26 cases in autumn, five in winter, six in spring but only two cases in summer), and no seasonal differences were detected between the different co-infections [23].
As seen in the present study Rotavirus is a most common detected antigen 40.74% for acute childhood diarrhea. These findings are consistent with other investigations in Izmir Rotavirus found 39.8% of 920 children [24]. We found Clostridium difficile infections(CDI) rate 13.88%. Some researchers find the incidence of CDI in the pediatric population appears to be increasing in US hospitals [25]. Our study has got some limitations because there is few investigations done in Erzurum before so our results are close for Rotaviruses and Adenoviruses [26].
Enteric viral pathogens were the most significant causative agents bacterial pathogens were also important contributors to pediatric diarrhea in Tripoli [27]. Resembling in Tripoli in this study viral pathogens detected 54.16% sample , bacterial pathogens detected 24.98% and parasitic agents detected 20.82% of all samples.
Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum are considered to be the most important diarrheal agents [28-32]. We search Entemoeba and Giardia enfections in our study. Giardia lamblia infections are very common throughout the world and are considered one of the main nonviral causes of diarrhea in industrialized countries [32]. For many years, microscopic examination of stool samples has been considered to be the "gold standard" for diagnosis of Entemoeba histolytica, Giardia lamblia and some parasites. Recently, more specific and sensitive alternative methods (PCR,ELISA) have been introduced for all these parasitic infections. We search these parasites with microscobic examination and we found Giardia spp 8.79% and Entemoeba spp 12.03% of all samples. These numbers shows us that parasitic infections are incontrovertible cause of acute diarrhea.
Conclusion
In coclusion analysis of viral antigens,bacterias and the parasites as a diarrheal agents in stool sample is important in 0-5 years old infants, in order to predict clinical outcome, prevent inapproprate antibiotic use and hospitalizations.
References
- Mahy BWJ, Regenmortel MV, Walker P, Majumder RD (2008) Encyclopedia of virology. (3rd edn), Enteric Viruses. Elsevier Ltd., Oxford, London, UK, p. 116.
- Black RE (1993) Epidemiology of diarrheal disease: implications for control by vaccines. Vaccine 11(2): 100-106.
- Barnes GL, Uren E, Stevens KB, Bishop RF (1998) Etiology of Acute Gastroenteritis in Hospitalized Children in Melbourne, Australia, from April 1980 to March 1993. J Clin Microbiol 36(1): 133-138.
- Huh JW, Moon SG, Lim YH (2009) A Survey of Intestinal Protozoan Infections among Gastroenteritis Patients during a 3-Year Period (20042006) in Gyeonggi-do (Province), South Korea. Korean J Parasitol 47(3): 303-305.
- Aranda Michel J, Giannella RA (1999) Acute diarrhea: a practical review. Am J Med 106(6): 670-676.
- Okeke IN, Ojo O, Lamikanra A, Kaper JB (2003) Etiology of acute diarrhea in adults in southwestern Nigeria. J Clin Microbiol 41(10): 4525-4530.
- Vargas M, Gascon J, Casals C, Schellenberg D, Urassa H, et al. (2004) Etiology of diarrhea in children less than five years of age in Ifakara, Tanzania. Am J Trop Med Hyg 70(5): 536-539.
- Langley JM, LeBlanc JC, Hanakowski M, Goloubeva O (2002) The role of Clostridium difficile and viruses as causes of nosocomial diarrhea in children. Infect Control Hosp Epidemiol 23(11): 660-664.
- Klein EJ, Boster DR, Stapp JR, Wells JG, Qin X, et al. (2006) Diarrhea etiology in a children's hospital emergency department: a prospective cohort study. Clin Infect Dis 43(7): 807-813.
- Denno DM, Shaikh N, Stapp JR, Qin X, Hutter CM, et al. (2012) Diarrhea etiology in a pediatric emergency department: a case control study. Clin Infect Dis 55(7): 897-904.
- Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, et al. (2008) Epidemiological features of Clostridium difficile-associated disease among inpatients in the United States, 2001-2006. Pediatrics 122(6): 1266-1270.
- Zilberberg MD, Shorr AF, Kollef MH (2008) Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000-2005. Pediatr Infect Dis J 27(12): 1111-1113.
- Toltzis P, Kim J, Dul M, Zoltanski J, Smathers S, et al. (2009) Presence of the epidemic North American pulsed field type 1 Clostridium difficile strain in hospitalized children. J Pediatr 154(4): 607-608.
- Suh KN, Gravel D, Mulvey MR, Moore DL, Miller M, et al. (2008) Clostridium difficile-associated infections in children admitted to acute care hospitals participating in the Canadian Nosocomial Infections Surveillance Program (CNISP), 2004-2005 [abstract 306]. In: Program of the 18th Annual Scientific Meeting of the Society of Healthcare Epidemiology of America. The Society, Orlando (FL), Arlington (VA), USA.
- McFarland LV, Brandmarker SA, Guandaline S (2000) Pediatric clostridium difficile: a phantom menace or clinical reality? J Pediatr Gastroenterol Nutr 31(3): 220-231.
- Snyder JD, Merson MH (1982) The magnitude of the global problem of acute diarrheal disease: a review of active surveillance data. Bull World Health Organ 60(4): 605-613.
- Bryce J, Boschi PC, Shibuya K, Black RE (2005) WHO estimates of the causes of death in children. Lancet 365(9465): 1147-1152.
- Jamison DT, Feachem RG, Makgoba MW, Boss ER, Baingana EK, et al. (2006) Disease and mortality in sub-saharan Africa. 2nd edition. World Bank, Washington (DC), US.
- Bern C, Martines J, de Zoysa I, Glass RI (1992) The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ 70(6): 705-714.
- Kosek M, Bern C, Guerrant R (2003) The global burden of diarrheal disease, as estimated from studies published Between 1992 and 2000. Bull World Health Organ 81(3): 197-204.
- Guerrant R, Hughes J, Lima N, Crane J (1990) Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev Infect Dis 12(1): 41-50.
- Friesema IHM, Boer DRF, Duizer E, Kortbeek LM, Notermans DW, et al. (2012) Etiology of acute gastroenteritis in children requiring hospitalization in the Netherlands. Eur J Clin Microbiol Infect Dis 31(4): 405-415.
- Roma'n E, Wilhelmi I, Colomina J, Villar J, Cilleruelo ML, et al. (2003) Fauquier acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. AS Journal of Medical Microbiology 52(5): 435-440.
- Kurugöl Z, Geylani S, Karaca Y, Umay F, Erensoy S, et al. (2003) The rotavirus gastroenteritis among children under five years of age in Izmir Turkey. Turk J Pediatr 45(4): 290-294.
- Vindigni SM, Shane AL (2010) Clostridium difficile infections among hospitalized children, United States, 1997-2006. Emerg Infect Dis 16(10): 1651.
- Balkan CE, Celebi D, Celebi Ö, Altoparlak Ü (2012) Investigation of rotavirus and adenovirus frequency among 0-5 years old children with acute gastroenteritis in erzurum,turkey. Journal of Turkish Society of Microbiology 42(2): 51-54.
- Rhouma A, Klena JD, Krema Z, Abobker AA, Treesh K, et al. (2011) Enteric pathogens associated with childhood diarrhea in Tripoli-Libya. Am J Trop Med Hyg 84(6): 886-891.
- Amin OM (2002) Seasonal prevelance of intestinal parasites in the United States during 2000. Am J Trop Med Hyg 66(6): 799-803.
- Current WL, Garcia S (1991) Cryptosporidiosis. Clin Microbiol Rev 4(3): 325-358.
- Wit DMAS, Koopmans MPG, Kortbeek LM, Leeuwen VNJ, Vinje J, et al. (2001) Etiology of gastroenteritis in sentinel general practices in the Netherlands. Clin Infect Dis 33(3): 280-288.
- Nichols GL (2000) Food-borne protozoa. Br Med Bull 56(1): 209-235.
- Thompson RCA, Hopkins RM, Homan WL (2000) Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol Today 16(5): 210-213.
© 2017 Çiğdem Eda Balkan, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






