- Submissions

Full Text
Clinical Research in Animal Science
Impact of Postbiotics on Ruminant Health and Productivity
Ishaya U Gadzama1*, Qazal Hina2, Isaac M Mugweru3, Methun C Dey4 and Abdullateef A Idris5
1School of Animal and Veterinary Sciences, University of Adelaide, Australia
2Department of Animal Nutrition, University of Veterinary and Animal Sciences, Pakistan
3Department of Animal Sciences, College of Agriculture and Natural Resources (COANRE), Jomo Kenyatta University of Agriculture and Technology, Kenya
4Institute of Livestock Science and Technology, Bangladesh
5Department of Animal Production, Faculty of Agriculture, University of Jos, Nigeria
*Corresponding author:Ishaya Usman Gadzama, School of Animal and Veterinary Sciences, University of Adelaide, Roseworthy, SA, 53711, Australia
Submission: July 07, 2025;Published: August 20, 2025

ISSN: 2770-6729Volume4 - Issue 1
Abstract
The increasing global demand for sustainable livestock production necessitates alternatives to antibiotic growth promoters, with postbiotics emerging as a viable solution due to their stability, safety and bioactive properties. This review synthesizes research on postbiotics-non-viable microbial components or metabolites, such as those derived from Saccharomyces Cerevisiae Fermentation Products (SCFP), Aspergillus oryzae and Lactobacillus spp. and their effects on ruminant health and productivity. Findings indicate that postbiotics enhance rumen fermentation by stabilizing pH, increasing volatile fatty acid production and modulating microbial populations, particularly fibrolytic bacteria such as Ruminococcaceae and Lachnospiraceae. Immunomodulatory benefits include reduced inflammatory markers (e.g., IL-6, TNF-α) and improved gut barrier function, achieved through the upregulation of tight junction proteins. Species- and dose-dependent responses are evident, with dairy cows showing improved nutrient digestibility and immunity, while beef cattle exhibit variable outcomes in rumen fermentation. Postbiotics also demonstrate antimicrobial effects, reducing pathogens like Salmonella and Staphylococcus aureus. Despite promising results, efficacy depends on the formulation, dosage and the animal’s physiological stage. This review highlights postbiotics as a strategic tool to enhance ruminant performance while aligning with One Health principles, though further research is needed to optimize their application across production systems.
Keywords:Postbiotics; Ruminant nutrition; Saccharomyces cerevisiae; Rumen fermentation; Immunomodulation; Antimicrobial resistance; Gut health; Feed additives; Sustainable agriculture
Introduction
The global livestock sector is under increasing pressure to meet the rising demand for animal-derived protein while addressing critical challenges related to Antimicrobial Resistance (AMR), feed efficiency and environmental sustainability [1-3]. Historically, antibiotics have been widely used in animal production not only for disease control but also as growth promoters [4-6]. However, the emergence of antimicrobial-resistant pathogens has led to stringent regulatory restrictions, including the European Union’s ban on antibiotic growth promoters in 2006 [7,8] and the U.S. FDA’s Veterinary Feed Directive in 2017 [9]. These measures highlight the urgent need for sustainable alternatives that enhance animal health and productivity without contributing to AMR.
One of the major challenges in modern ruminant production is the disruption of gut microbiota due to high-concentrate diets, which can lead to Subacute Ruminal Acidosis (SARA), liver abscesses and systemic inflammation [10]. Such conditions not only impair animal performance but also increase susceptibility to pathogens such as Salmonella and Fusobacterium [1,9,11,12]. In this context, postbiotics are gaining significant attention as a novel category of “biotics,” offering a promising alternative to traditional antibiotics in livestock production, particularly for ruminants. The International Scientific Association of Probiotics and Prebiotics (ISAPP) defines postbiotics as “a preparation of inanimate microorganisms and/ or their components that confers a health benefit on the host” [13]. Postbiotics are generated through the fermentation of probiotics, where these probiotics produce bioactive compounds during anaerobic fermentation [14]. Extraction methods include centrifugation, ultrafiltration, chromatography and mass spectrometry [15]. This means they are non-living products, often derived from microbial fermentations, comprising cellular components, metabolites and fermentation end products [13,16]. Unlike live probiotics, postbiotics are more stable and safer, as they do not contain live microorganisms, thereby reducing the risk of gut-to-blood bacterial translocation or the acquisition of antibioticresistant genes [17,18]. They also have a longer shelf life and are not inactivated by chemicals or drugs [6], making them particularly suitable for inclusion in animal feed [1,19].
The multifaceted chemical composition of postbiotics highlights their profound biological relevance in ruminant nutrition and gut health [20]. Postbiotics encompass a wide range of bioactive compounds and metabolites, each contributing distinct functional attributes that influence host physiology, microbial ecology and immune modulation [21,22]. The strategic application of postbiotics in ruminant diets hinges on a nuanced understanding of their chemical constituents and mechanistic pathways. Short-Chain Fatty Acids (SCFAs), particularly acetate, propionate and butyrate (Figure 1), serve as essential energy substrates for enterocytes and exhibit systemic anti-inflammatory effects, thereby enhancing intestinal barrier integrity-a critical factor in mitigating metabolic stress in high-producing ruminants [21,23-25]. Organic acids, including lactic and phenylacetic acids, exert a bacteriostatic effect by modulating luminal pH, thus selectively inhibiting pathogenic colonization while fostering commensal microbiota proliferation [26,27]. Exopolysaccharides (EPS) and bacteriocins further exemplify the dual role of postbiotics in pathogen exclusion and immune priming. EPS such as β-glucans enhance mucosal immunity through receptor-mediated signalling [28,29], while bacteriocins, such as nisin, provide targeted antimicrobial activity without disrupting symbiotic microbial consortia [2,30,31]. The presence of B vitamins and antioxidant enzymes within postbiotic matrices (Figure 1) also suggests a synergistic role in ameliorating oxidative stress, a common constraint in intensive production systems [28,32,33].
Fgure 1:The diverse and complex chemical composition of postbiotics underpins their wide range of biological activities.
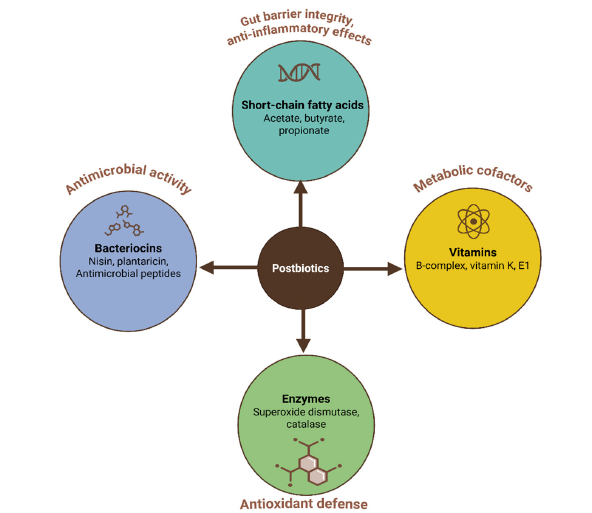
The efficacy of postbiotics depends on their source and compositional profile [11]. For instance, Saccharomyces Cerevisiae Fermentation Products (SCFP) have been extensively validated in dairy cattle, where their metabolite-rich composition improves fibre digestibility, enhances feed efficiency, reduces lactic acid accumulation, and promotes beneficial microbial populations in the rumen [34,35] and lactation performance [36-39]. Similarly, Lactobacillus plantarum RG14 metabolites demonstrate significant benefits in young ruminants by improving nutrient digestibility and inhibiting pathogenic bacteria through lowered intestinal pH and the formation of protective biofilms [22], highlighting the strain-specific nature of postbiotic effects [22]. Recent research has expanded our understanding of postbiotic applications across different ruminant species and production stages [1,5,17,40]. In dairy cows, SCFP supplementation has been shown to mitigate SARA by stabilizing ruminal pH and enhancing Volatile Fatty Acid (VFA) production [41,42]. In calves, postbiotics improve immune function and reduce diarrhea incidence [5,43-45], while in beef cattle, they enhance feed efficiency and liver health [46]. Additionally, postbiotics exhibit immunomodulatory properties, reducing systemic inflammation and oxidative stress markers such as Serum Amyloid A (SAA) and Lipopolysaccharide-Binding Protein (LBP) [17,32,39,47-52]. Although significant progress has been made, there are still gaps in optimizing postbiotic formulations for various production systems and in understanding their long-term effects on rumen microbiome dynamics. The primary objective of this review is to synthesize existing knowledge on postbiotic applications in ruminant nutrition, focusing on their impact on rumen fermentation and animal health. This work aims to contribute to the development of sustainable strategies that enhance animal health, productivity, and food safety while aligning with the “One Health” paradigm.
Effects of postbiotics on rumen fermentation and animal health
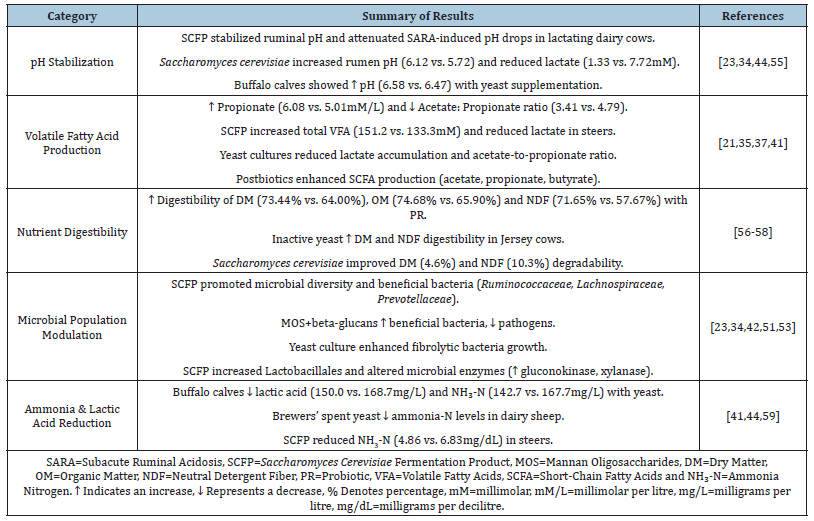
The efficacy of Saccharomyces cerevisiae fermentation products in stabilizing ruminal pH during dietary stress is well-documented (Table 1). In lactating Holstein cows subjected to SARA challenges, SCFP supplementation (14-38g/d) consistently mitigated pH fluctuations and lactate accumulation, while enriching fibrolytic taxa (Ruminococcaceae, Lachnospiraceae) essential for fibre degradation [34,42,53,54]. This microbial shift correlated with increased acetate production and reduced proteolytic activity, enhancing nitrogen utilization efficiency. SCFP’s benefits were dose-dependent; for example, higher doses (38g/d of SCFPb- 2X) amplified rumen resilience during high-starch feeding by attenuating propionate metabolism and stabilizing the Firmicutes: Bacteroidetes ratio [34,42]. On the other hand, steers receiving a combination of liquid (11mL/100kg BW) and dry SCFP (12g/d) showed a 28.8% decrease in ruminal NH₃-N, accompanied by an increase in valerate, indicating enhanced peptide metabolism [41,52]. Such findings accentuate SCFP’s role in optimizing fermentation stoichiometry, though responses vary with delivery method and basal diet composition [55-59].
Table 1:Effects of postbiotics on rumen fermentation parameters.
Immunomodulatory and anti-inflammatory mechanisms
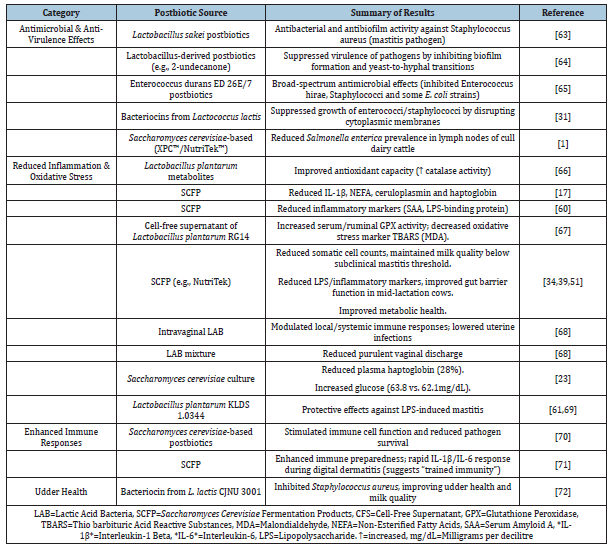
Beyond rumen modulation, SCFP exerts systemic immunoregulatory effects (Table 2). Transition cows supplemented pre-and postpartum (19g/d) exhibited reduced Serum Amyloid A (SAA) and LPS-binding protein, suggesting mitigation of endotoxin translocation [60]. This aligns with Guo et al. [34], who found that SCFPb-2X (38g/d) downregulated pro-inflammatory cytokines (IL-6, TNF-α) by 25-30% during SARA, while elevating the antiinflammatory cytokine IL-10. The mechanistic link involves enhanced gut barrier integrity, as evidenced by the upregulation of tight junction proteins (occludin, claudin-1) in mid-lactation cows [51]. Similarly, Aspergillus oryzae fermentation extract (3-6g/d) reduced plasma LBP and IL-6 in lactating Holsteins, corroborating the anti-inflammatory potential of fungal metabolites [32]. Intriguingly, Ferguson et al. [61] reported a reduced incidence of mastitis with SCFP, while Thomas et al. [62] observed no such effect-A discrepancy potentially attributable to herd health status or basal diet differences (Table 2).
Table 2:Effects of postbiotics on animal health and immune function.
Interaction between postbiotics and the rumen microbiome
Postbiotics are gaining significant attention as a novel category of “biotics,” offering a promising alternative to traditional antibiotics in livestock production, particularly for ruminants [63-66]. The interaction between postbiotics and the rumen microbiome has been the topic of several studies due to their potential to enhance ruminant productivity while mitigating environmental impacts [67]. Recent studies demonstrate that postbiotics exert species and stage-specific effects on rumen fermentation and microbial ecology. In goats, yeast-derived postbiotics (Probisan Ruminants) administered at 3.75g/d during late lactation increased propionate production by 21% and improved the acetate-to-propionate ratio, suggesting enhanced energy utilization [37,68,69]. Similarly, lambs supplemented with Lactobacillus plantarum RG14 postbiotics exhibited a selective reduction in Enterobacteriaceae without disrupting total bacterial populations, indicating targeted antimicrobial activity against potential pathogens [22,70-72]. These findings highlight the capacity of postbiotics to modulate microbial communities in a manner that supports host health and metabolic efficiency. In young ruminants, postbiotic supplementation has shown promise in boosting immune defences. Calves receiving Saccharomyces Cerevisiae Fermentation Products (SCFP) at 1-2g/ d demonstrated improved resistance to respiratory and enteric pathogens, with notable reductions in Salmonella-induced diarrhea and lung pathology [73,74]. However, responses vary with the dietary context, as evidenced by beef heifers on high-grain diets showing improved fermentation profiles with SCFP, whereas midfattening Angus steers exhibited no significant changes in rumen parameters [75,76].
This highlights the importance of dosage, dietary composition and physiological stage in determining the efficacy of postbiotics. Beyond immediate performance benefits, postbiotics influence rumen microbial ecology in ways that enhance long-term feed efficiency [20,23,77]. Studies in newly weaned lambs have revealed that postbiotics increase weight gain, nutrient digestibility, and populations of fibrolytic bacteria, while reducing protozoa and methanogens [22]. In vitro work further supports these observations, demonstrating that postbiotics from L. plantarum RG14 enhance organic matter digestibility and volatile fatty acid production without compromising rumen pH [78]. Such improvements in fermentation efficiency are critical for optimizing feed conversion in production systems. A particularly compelling aspect of postbiotic supplementation is its potential to reduce methane emissions. By suppressing methanogen populations, postbiotics directly lower methane output [22]. Additionally, Saccharomyces cerevisiae postbiotics promote microbial stability, fostering lactate-utilizing and fibrolytic bacteria while mitigating subacute ruminal acidosis [42]. This stabilization of rumen microbiota not only improves animal health but also redirects metabolic hydrogen toward propionate synthesis rather than methanogenesis [77,79]. The resulting shift in fermentation pathways aligns with broader goals of sustainable livestock production. The mechanisms underlying these effects involve intricate microbial interactions. Postbiotics modulate the rumen microbiome’s composition and functional dynamics, enhancing fermentation efficiency while reducing environmental pollutants [80]. As research progresses, a deeper understanding of these interactions will enable more targeted applications, ensuring that postbiotics are utilized optimally across different production systems. For farmers and nutritionists, these findings offer practical strategies to improve both animal performance and environmental sustainability.
Conclusion
The growing body of research stresses the potential of postbiotics as a viable alternative to antibiotics in ruminant nutrition, offering benefits in rumen fermentation, immune modulation and overall animal health. Postbiotics, particularly those derived from Saccharomyces cerevisiae and Lactobacillus spp., demonstrate consistent improvements in rumen pH stability, volatile fatty acid production, and nutrient digestibility, while mitigating subacute ruminal acidosis and reducing pathogenic bacterial loads. Their immunomodulatory effects, including reduced inflammatory markers and enhanced gut barrier function, further support their role in promoting animal health without the risks associated with live probiotics or antimicrobial resistance. However, the efficacy of postbiotics varies depending on factors such as dosage, animal species and physiological stage, highlighting the need for standardized protocols. While current findings are promising, further large-scale, long-term studies are necessary to validate these effects across diverse production systems. The integration of postbiotics into ruminant diets aligns with sustainable livestock production goals, offering a science-backed strategy to enhance productivity while addressing global concerns over antimicrobial resistance.
Future directions and research gaps
Despite the demonstrated benefits, critical gaps remain in postbiotic research, particularly regarding optimal dosing, speciesspecific responses and long-term metabolic impacts. Future studies should prioritize in vivo trials evaluating postbiotic efficacy in methane mitigation, feed efficiency and immune function under varying dietary conditions. Additionally, the economic feasibility of large-scale postbiotic production must be assessed to facilitate industry adoption. Mechanistic insights into rumen-microbepostbiotic interactions, particularly in relation to methanogen suppression and volatile fatty acid dynamics, warrant deeper investigation. Standardized methodologies for postbiotic characterization and application will be essential to maximize their potential in sustainable ruminant production systems.
References
- Edache SE, Horton V, Dewsbury DM, George LA, Shi X, et al. (2024) Evaluation of a postbiotic on salmonella enterica prevalence, serotype diversity and antimicrobial resistance in the subiliac lymph nodes of cull dairy cattle. J Food Prot 87(12): 100375.
- Odey TOJ, Tanimowo WO, Afolabi KO, Jahid IK, Reuben RC (2024) Antimicrobial use and resistance in food animal production: Food safety and associated concerns in Sub-Saharan Africa. Int Microbiol 27(1):1-23.
- Gadzama IU, Ray S, Méité R, Mugweru IM, Gondo T, et al. (2025) Chlorella vulgaris as a livestock supplement and animal feed: A comprehensive review. Animals 15(16): 879.
- Castanon J (2007) History of the use of antibiotics as growth promoters in European poultry feeds. Poultry Sci 86(11): 2466-2471.
- Centeno-Martinez RE, Dong W, Klopp RN, Yoon I, Boerman JP, et al. (2023) Effects of feeding Saccharomyces cerevisiae fermentation postbiotic on the fecal microbial community of Holstein dairy calves. Anim Microbiome 5(1): 13.
- Shrika K, Sabarish S, Dharumadurai D (2025) Chapter 29-postbiotic metabolites in livestock feeding. Postbiotics, Academic Press, Massachusetts, USA, pp. 531-541.
- Millet S, Maertens L (2011) The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet J 187(2): 143-144.
- Commission E (2005) Ban on antibiotics as growth promoters in animal feed enters into effect.
- Sarkar S, Okafor CC (2024) Impact of veterinary feed directive rules changes on the prevalence of antibiotic resistance bacteria isolated from cecal samples of food-producing animals at US slaughterhouses. Pathogens 13(8): 631.
- Callaway TR, Koyun O, Corcionivoschi N, Baloyi JJ, Ateba C, et al. (2023) Practical applications of probiotics in beef cattle production. Direct-Fed Microbials and Prebiotics for Animals, pp 301-322.
- Chaney WE, McBride H, Girgis G (2023) Effect of a saccharomyces cerevisiae postbiotic feed additive on salmonella enteritidis colonization of cecal and ovarian tissues in directly challenged and horizontally exposed layer pullets. Animals 13(7): 1186.
- Chaney WE, Naqvi SA, Gutierrez M, Gernat A, Johnson TJ, et al. (2022) Dietary inclusion of a saccharomyces cerevisiae-derived postbiotic is associated with lower salmonella enterica burden in broiler chickens on a commercial farm in Honduras. Microorganisms 10(3): 544.
- Salminen S, Collado MC, Endo A, Hill C, Lebeer S, et al. (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 18(9): 649-667.
- Homayouni Rad A, Pouragha B, Houshyar J, Soleimani RA, Kazemi S, et al. (2025) Postbiotic application: A Review on extraction, purification and characterization methods. Food Bioprocess Technol 18(5): 4153-4174.
- Bogdanović M, Mladenović D, Mojović L, Djuriš J, Djukić-Vuković A (2024) Intraoral administration of probiotics and postbiotics: An overview of microorganisms and formulation strategies. Braz J Pharm Sci 60:
- Nataraj BH, Ali SA, Behare PV, Yadav H (2020) Postbiotics-parabiotic: The new horizons in microbial biotherapy and functional foods. Microb Cell Fact 19(1): 168.
- Dai D, Kong F, Han H, Shi W, Song H, et al. (2024) Effects of postbiotic products from Saccharomyces cerevisiae fermentation on lactation performance, antioxidant capacity and blood immunity in transition dairy cows. J Dairy Sci 107(12): 10584-10598.
- Wegh CAM, Geerlings SY, Knol J, Roeselers G, Belzer C (2019) Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci 20(19): 4673.
- Zamojska D, Nowak A, Nowak I, Macierzynska-Piotrowska E (2021) Probiotics and postbiotics as substitutes of antibiotics in farm animals: A review. Animals 11(12): 3431.
- Coleman DN, Jiang Q, Lopes MG, Ritt L, Liang Y, et al. (2023) Feeding a Saccharomyces cerevisiae fermentation product before and during a feed restriction challenge on milk production, plasma biomarkers and immune function in Holstein cows. J Anim Sci 101:
- Marlida Y, Shun TJ, Syofyan S, Ardani LR, Anggraini L (2024) Postbiotic studies of mixed cultures of Schleifer lactobacillus harbinensis LH991 and Pichia kudriavzevii B-5P produced by in vitro rumen producing short-chain fatty acid. Vet World 17(11): 2694-2700.
- Izuddin WI, Loh TC, Foo HL, Samsudin AA, Humam AM (2019) Postbiotic plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci Rep 9(1): 9938.
- Dias ALG, Freitas JA, Micai B, Azevedo RA, Greco LF, et al. (2018) Effects of supplementing yeast culture to diets differing in starch content on performance and feeding behavior of dairy cows. J Dairy Sci 101(1): 186-200.
- Wu D, Wu C, Shao K, Zhang Z, Wang D, et al. (2024) Interactions of starter starch and sodium butyrate for post weaned dairy calves: Growth performance, blood indices and inflammation. Anim Feed Sci Technol 309: 115898.
- Wu D, Zhang Z, Song Q, Jia Y, Qi J, et al. (2024) Modulating gastrointestinal microbiota in preweaning dairy calves: Dose-dependent effects of milk-based sodium butyrate supplementation. Microorganisms 12(2): 333.
- Rafique N, Jan SY, Dar AH, Dash KK, Sarkar A, et al. (2023) Promising bioactivities of postbiotics: A comprehensive review. J Agric Food Res 14: 100708.
- Abd El-Ghany WA (2024) Applications of organic acids in poultry production: An updated and comprehensive review. Agriculture 14(10):
- Ansari F, Pourjafar H, Samakkhah SA, Mirzakhani E (2024) An overview of probiotic camel milk as a nutritional beverage: Challenges and perspectives. Food Sci Nutr 12(9): 6123-6141.
- La Torre C, Plastina P, Abrego-Guandique DM, Caputo P, Oliviero Rossi C, et al. (2024) Characterization of exopolysaccharides isolated from donkey milk and its biological safety for skincare applications. Polysaccharides 5(3): 493-503.
- Fijan S, Turk DM, Pogačar M, Koren M (2024) Bacteriocins or microbial peptides produced by probiotics. Postbiotics: Health and Industry, pp 241-263.
- Lauková A, Dvorožňáková E, Vargová M, Ščerbová J, Focková V, et al. (2023) The bacteriocin-like inhibitory substance producing Lacticaseibacillus paracasei lpa 12/1 from raw goat milk, a potential additive in dairy products. Appl Sci 13(22).
- Kaufman JD, Seidler Y, Bailey HR, Whitacre L, Bargo F, et al. (2021) A postbiotic from Aspergillus oryzae attenuates the impact of heat stress in ectothermic and endothermic organisms. Sci Rep 11(1): 6407.
- Papatsiros VG, Eliopoulos C, Voulgarakis N, Arapoglou D, Riahi I, et al. (2023) Effects of a multi-component mycotoxin-detoxifying agent on oxidative stress, health and performance of sows. Toxins 15(9): 580.
- Guo J, Zhang Z, Guan LL, Yoon I, Plaizier JC, et al. (2024) Postbiotics from Saccharomyces cerevisiae fermentation stabilize microbiota in rumen liquid digesta during grain-based Subacute Ruminal Acidosis (SARA) in lactating dairy cows. J Anim Sci Biotechnol 15(1): 101.
- Williams PE, Tait CA, Innes GM, Newbold CJ (1991) Effects of the inclusion of yeast culture (Saccharomyces cerevisiae plus growth medium) in the diet of dairy cows on milk yield and forage degradation and fermentation patterns in the rumen of steers. Journal of animal science 69(7): 3016-3026.
- Faraz A, Buzdar HQ, Waheed A, Hussain SM, Rahman SU, et al. (2025) Milk production profile of Barela camel (Camelus dromedarius) supplemented with postbiotics in a semi-intensive management system: Pilot study. Front Vet Sci 12: 1576912.
- Hansen HH, El-Bordeny NE, Ebeid HM (2017) Response of primiparous and multiparous buffaloes to yeast culture supplementation during early and mid-lactation. Anim Nutr 3(4): 411-418.
- Fernandez C, Romero T, Badiola I, Diaz-Cano J, Sanzol G, et al. (2023) Postbiotic yeast fermentation product supplementation to lactating goats increases the efficiency of milk production by enhancing fiber digestibility and ruminal propionate and reduces energy losses in methane. J Anim Sci 101:
- Jiang Q, Sherlock DN, Elolimy AA, Vailati-Riboni M, Yoon I, et al. (2023) Impact of a Saccharomyces cerevisiae fermentation product during an intestinal barrier challenge in lactating Holstein cows on ileal microbiota and markers of tissue structure and immunity. J Anim Sci 101:
- Kamalamma, Krishnamoorthy U, Krishnappa P (1996) Effect of feeding yeast culture (Yea-sacc1026) on rumen fermentation in vitro and production performance in crossbred dairy cows. Anim Feed Sci Technol 57(3): 247-256.
- Odunfa OA, Dhungana A, Huang Z, Yoon I, Jiang Y (2024) Effects of a liquid and dry Saccharomyces cerevisiae fermentation product feeding program on ruminal fermentation, total tract digestibility and plasma metabolome of Holstein steers receiving a grain-based diet. J Anim Sci 102:
- Guo J, Zhang Z, Guan LL, Zhou M, Yoon I, et al. (2024) Postbiotics from Saccharomyces cerevisiae fermentation stabilizes rumen solids microbiota and promote microbial network interactions and diversity of hub taxa during grain-based Subacute Ruminal Acidosis (SARA) challenges in lactating dairy cows. Front Microbiol 15: 1409659.
- Cao Z, Xiao J, Alugongo GM, Ji S, Wu Z, et al. (2019) Effects of saccharomyces cerevisiae fermentation products on the microbial community throughout the gastrointestinal tract of calves. Animals 9(1): 4.
- Kumar U, Sareen VK, Singh S (1997) Effect of yeast culture supplement on ruminal microbial populations and metabolism in buffalo calves fed a high roughage diet. J Sci Food Agric 73(2): 231-236.
- Koul V, Kumar U, Sareen VK, Singh S (1998) Mode of action of yeast culture (YEA-SACC 1026) for stimulation of rumen fermentation in buffalo calves. J Sci Food Agric 77(3): 407-413.
- Bettini S, Perini F, Colombi D, Ghilardi M, Trabalza-Marinucci M, et al. (2025) Assessing the impact of biotics on the ruminal microbiome to enhance sustainability, welfare and performance in beef cattle: Highlighting the omics approach. Ital J Anim Sci 24(1): 660-676.
- Fan Z, Jia W (2023) Lactobacillus casei-derived postbiotics elevate the bio accessibility of proteins via allosteric regulation of pepsin and trypsin and introduction of endopeptidases. J Agric Food Chem 71(28): 10647-10669.
- Rius AG, Kaufman JD, Li MM, Hanigan MD, Ipharraguerre IR (2022) Physiological responses of Holstein calves to heat stress and dietary supplementation with a postbiotic from Aspergillus oryzae. Sci Rep 12(1): 1587.
- Jiang J, Xi M, Wang B, Zhang R (2024) Effects of methionine on liver health of ruminants and its mechanisms. Chin J Anim Nutr 36(11): 6853-6863.
- Jiang Q, Galvão MC, Alharthi AS, Alhidary IA, Gionbelli MP, et al. (2024) Feed restriction in angus steers impacts ruminal bacteria, its metabolites and causes epithelial inflammation. Ruminants 4(3): 387-405.
- Jiang Q, Palombo V, Sherlock DN, Vailati-Riboni M, D Andrea M, et al. (2023) Alterations in ileal transcriptomics during an intestinal barrier challenge in lactating Holstein cows fed a Saccharomyces cerevisiae fermentation product identify potential regulatory processes. J Anim Sci 101:
- Jiang Y, Dhungana A, Odunfa OA, McCoun M, McGill J, et al. (2025) Effects of Saccharomyces cerevisiae fermentation product on ruminal fermentation, total tract digestibility, blood proinflammatory cytokines and plasma metabolome of Holstein steers fed a high-grain diet. Transl Anim Sci 9: txaf058.
- Guo J, Zhao Y, Guo W, Sun Y, Zhang W, et al. (2025) Effects of Lactobacillus paracei JY062 postbiotic on intestinal barrier, immunity and gut microbiota. Nutrients 17(7): 1272.
- Amanzougarene Z, Tejeda MP, Calvo H, De la Fuente G, Fondevila M (2020) Microbial fermentation of starch-or fiber-rich feeds added with dry or pre-activated Saccharomyces cerevisiae studied in vitro under conditions simulating high-concentrate feeding for ruminants. J Sci Food Agric 100(5): 2236-2243.
- Guo J, Xu L, Khalouei H, Fehr K, Senaratne V, et al. (2022) Saccharomyces cerevisiae fermentation products reduce bacterial endotoxin concentrations and inflammation during grain-based subacute ruminal acidosis in lactating dairy cows. J Dairy Sci 105(3): 2354-2368.
- Vicente F, Campo-Celada M, Menendez-Miranda M, Garcia-Rodriguez J, Martinez-Fernandez A (2024) Effect of postbiotic supplementation on nutrient digestibility and milk yield during the transition period in dairy cows. Animals 14(16): 2359.
- Gandra JR, Takiya CS, Gandra ERS, Pedrini CA, Oliveira ER, et al. (2024) Feeding live or inactive yeast to primiparous Jersey cows: Nutrient digestibility, rumen fermentation and milk fatty acid profile. NZJ Agric Res 68(5): 961-975.
- De Poppi AC, Lazzari G, Gomes ALM, Do Prado RM, De Almeida RTR, et al. (2021) Effects of feeding a live yeast on rumen fermentation and fiber degradability of tropical and subtropical forages. J Sci Food Agric 101(15): 6220-6227.
- Oancea AG, Dragomir C, Untea A, Saracila M, Turcu R, et al. (2023) The effects of Brewer's Spent Yeast (BSY) inclusion in dairy sheep's diets on ruminal fermentation and milk quality parameters. Agriculture 13(8):
- Sivinski S, Meier K, Mamedova L, Saylor B, Shaffer J, et al. (2022) Effect of Saccharomyces cerevisiae fermentation product on oxidative status, inflammation and immune response in transition dairy cattle. J Dairy Sci 105(11): 8850-8865.
- Ferguson J, Sattler M, Hanson D, Davis C, Edrington T, et al. (2018) Feeding Nutri Tek reduces linear scores and clinical mastitis cases. J Dairy Sci 101(Suppl 2): 135.
- Thomas M, Serrenho RC, Puga SO, Torres JM, Puga SO, et al. (2023) Effect of feeding a Saccharomyces cerevisiae fermentation product to Holstein cows exposed to high temperature and humidity conditions on milk production performance and efficiency-A pen-level trial. J Dairy Sci 106(7): 4650-4665.
- Sevin S, Karaca B, Haliscelik O, Kibar H, OmerOglou E, et al. (2021) Postbiotics secreted by Lactobacillus sakei EIR/CM-1 isolated from cow milk microbiota, display antibacterial and antibiofilm activity against ruminant mastitis-causing pathogens. Ital J Anim Sci 20(1): 1302-1316.
- Rajasekharan SK, Shemesh M (2022) The bacillary postbiotics, including 2-undecanone, suppress the virulence of pathogenic microorganisms. Pharmaceutics 14(5): 962.
- Laukova A, Fockova V, Madar M, Belzecki G, Miltko R, et al. (2025) Fecal strains Enterococcus mundtii from wild ruminants, their safety and postbiotic potential. Vet Res Commun 49(3): 141.
- Shi CX, Yang SR, Li YQ, Wang HL, Min SN, et al. (2025) Saccharomyces cerevisiae supplementation improves growth performance and heat stress tolerance in angus steers. Agriculture 15(4):
- Izuddin WI, Humam AM, Loh TC, Foo HL, Samsudin AA (2020) Dietary postbiotic lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants 9(3): 250.
- Adnane M, Whiston R, Tasara T, Bleul U, Chapwanya A (2024) Harnessing vaginal probiotics for enhanced management of uterine disease and reproductive performance in dairy cows: A conceptual review. Animals 14(7): 1073.
- Zhumakayeva A, Zhubatkanova A, Asauova Z, Tokayeva M, Kemeshov Z (2023) Efficiency of probiotic culture consortium application for disinfection of dairy farm premises and prevention of mastitis in cows. J Adv Vet Anim Res 10(2): 185-195.
- Jensen G, Patterson K, Yoon I (2008) Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp Immunol Microbiol Infect Dis 31(6): 487-500.
- Henige M, Anklam K, Aviles M, Buettner J, Henschel S, et al. (2024) The effect of saccharomyces cerevisiae fermentation product supplementation on pro-inflammatory cytokines in Holstein Friesian cattle experimentally inoculated with digital dermatitis. Animals 14(22): 3260.
- Kim SG, Lee YD, Park JH, Moon GS (2019) Synergistic inhibition by bacteriocin and bacteriophage against Staphylococcus aureus. Food Sci Anim Resour 39(6): 1015-1020.
- Brewer MT, Anderson KL, Yoon I, Scott MF, Carlson SA (2014) Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet Microbiol 172(1-2): 248-255.
- Mahmoud AH, Slate JR, Hong S, Yoon I, McGill JL (2020) Supplementing a Saccharomyces cerevisiae fermentation product modulates innate immune function and ameliorates bovine respiratory syncytial virus infection in neonatal calves. J Anim Sci 98(8): skaa252.
- Shi CX, Yang SR, Li YQ, Wang HL, Min SN, et al. (2025) Saccharomyces cerevisiae supplementation improves growth performance and heat stress tolerance in angus steers. Agriculture 15(4):
- Shen Y, Wang H, Ran T, Yoon I, Saleem AM, et al. (2018) Influence of yeast culture and feed antibiotics on ruminal fermentation and site and extent of digestion in beef heifers fed high grain rations. J Anim Sci 96(9): 3916-3927.
- Badhan A, Wang Y, Terry S, Gruninger R, Guan LL, et al. (2025) Invited review: Interplay of rumen microbiome and the cattle host in modulating feed efficiency and methane emissions. J Dairy Sci 108(6): 5489-5501.
- Izuddin WI, Loh TC, Samsudin AA, Foo HL (2018) In vitro study of postbiotics from Lactobacillus plantarum RG14 on rumen fermentation and microbial population. Rev Bras Zootec 47:
- Faiz-Ul Hassan, Muhammad A Arshad, Hossam M Ebeid, Muhammad S Rehman, Muhammad S Khan, et al. (2020) Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet-microbe interaction. Front Vet Sci 7: 575801.
- Welch CB, Ryman VE, Pringle TD, Lourenco JM (2022) Utilizing the gastrointestinal microbiota to modulate cattle health through the microbiome-gut-organ axes. Microorganisms 10(7): 1391.
© 2025 Ishaya U Gadzama. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






