- Submissions

Full Text
Aspects in Mining & Mineral Science
Most Common Misprints in Definitions of Zeldovich Number Fatally Affecting Preliminary Estimates of Combustion Thermal Activity
I A Filimonov*
Merzhanov Institute of Structural Macrokinetics and Materials Science (ISMAN) RAS, Russia
*Corresponding author:I A Filimonov, Merzhanov Institute of Structural Macrokinetics and Materials Science (ISMAN) RAS, Academician Osipyan Str, 8, Chernogolovka, Moscow Region, 142432, Russia
Submission: September 16, 2024: Published: September 26, 2025

ISSN 2578-0255Volume14 Issue 2
Opinion
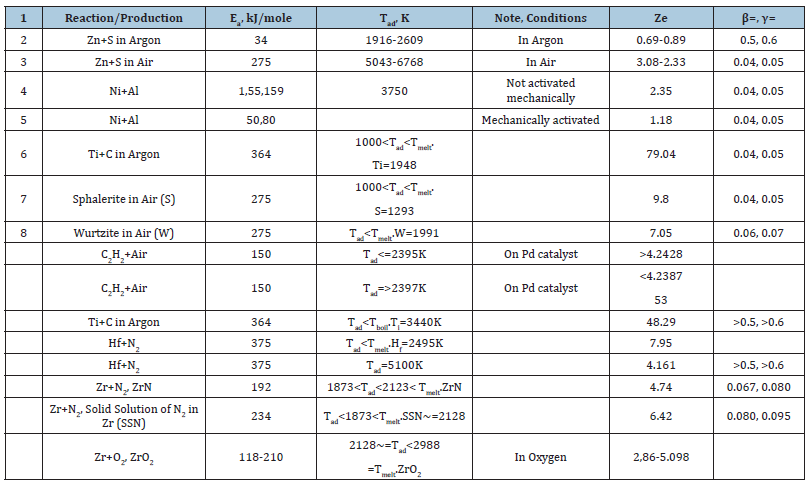
Any investigation concerning experimental data or computer modeling needs that the preliminary estimates have been already done. In the case of combustion thermal activity these estimates are based inevitably on the well-known Zeldovich number (Ze). Nevertheless, it turns out, that the existing Ze definitions are strongly different both from each other and from the correct version of them [1-3]. In the paper presented let’s consider the most common misprints in definitions of Ze and explain the contradictory conclusions they lead to. In accordance with the electronic encyclopedia (Wikipedia 2025) the first definition of Zeldovich number has been published in proceedings of the ICDERS meeting [4], at Poitiers 1983: 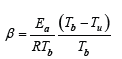 . Actually, 𝛽 is the wave parameter providing its existence (at 𝛽 ≪1 , 𝛾≪1, 𝑠ee Table 1)
. Actually, 𝛽 is the wave parameter providing its existence (at 𝛽 ≪1 , 𝛾≪1, 𝑠ee Table 1)
where,
𝛽 has been supposed to be the Ze number, while
𝐸𝑎 is the activation energy,
𝑇𝑏 is a burnt gas temperature,
𝑇𝑢 is an unburnt gas temperature.
Nevertheless, even earlier Zeldovich himself has written in his famous book (1) the following expression as definition of his number (introduced as z in the book): 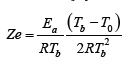 ,
,
At the same time A.G. Merzhanov in his monography (2) uses the adiabatic temperature 𝑇𝑎𝑑 instead of the actual combustion temperature 𝑇b (see (2)): 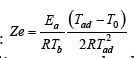 . To illustrate the huge difference between expressions (2) and (3) leading to completely contradictory conclusions, let’s consider the hydrogen-oxygen flame stability. It is well-known that there is practically no difference between 𝑇𝑏 𝑎𝑛𝑑 𝑇𝑎𝑑 in turbulent combustion [5-7]. In combustion synthesis 𝑇𝑎𝑑 corresponds to the complete conversion, while 𝑇𝑏 may be related with an incomplete transformation of the initial components [8]. Correspondingly, the turbulent combustion of premixed hydrogen-oxygen mixtures is absolutely stable (𝑍𝑒<𝑍𝑒𝑐𝑟=2+√5) while vice versa the hydrogen-oxygen flame with an incomplete
conversion (𝑍𝑒≥𝑍𝑒𝑐𝑟=2+ √5) oscillates and demonstrates unstable
propagation (see [3]). Thus, one have to understand that correct
definition of Ze is described by the expression (2) only. The work
presented above has been done under the state assignment for
ISMAN (125021201988-9).
. To illustrate the huge difference between expressions (2) and (3) leading to completely contradictory conclusions, let’s consider the hydrogen-oxygen flame stability. It is well-known that there is practically no difference between 𝑇𝑏 𝑎𝑛𝑑 𝑇𝑎𝑑 in turbulent combustion [5-7]. In combustion synthesis 𝑇𝑎𝑑 corresponds to the complete conversion, while 𝑇𝑏 may be related with an incomplete transformation of the initial components [8]. Correspondingly, the turbulent combustion of premixed hydrogen-oxygen mixtures is absolutely stable (𝑍𝑒<𝑍𝑒𝑐𝑟=2+√5) while vice versa the hydrogen-oxygen flame with an incomplete
conversion (𝑍𝑒≥𝑍𝑒𝑐𝑟=2+ √5) oscillates and demonstrates unstable
propagation (see [3]). Thus, one have to understand that correct
definition of Ze is described by the expression (2) only. The work
presented above has been done under the state assignment for
ISMAN (125021201988-9).
Table 1:Combustion reactions and the conditions of their activation.
References
- Zeldovich YB, Librovich VB, Makhviladze GM (1980) Mathematical theory of combustion and explosion. Nauka, Moscow, Russia, p. 263.
- Merzhanov AG (2000) Solid flame combustion. ISMAN p. 127.
- Vadchenko SG, Rogachev AS (2024) Evidence of an oscillating reaction during heating of TiH2 in air. Internat J of SHS 33(3): 245-248.
- (1945) Hinshelwood.
- Lipatnikov AN, Sabelnikov VA (2020) An extended flamelet-based presumed probability density function for predicting mean concentrations of various species in premixed turbulent flames. Internat J of Hydrogen Energy 45(55): 31162-31178.
- Lipatnikov AN, Sabelnikov VA, Hernandez-Perez FE, Song W, Im HG (2021) Prediction of mean radical concentrations in lean hydrogen-air turbulent flames at different Karlovitz numbers adopting a newly extended flamelet-based presumed PDF. Combustion and Flame 226: 248-259.
- Poinsot T, Veynante D (2005) Theoretical and numerical combustion. Edwards. (2nd edn).
- Gullis CF, Hinshelwood CN (1945) The mechanism of the hydrogen-oxygen reaction. IV. The activation energy of the initiating process. Publication of F R S.
© 2025 I A Filimonov*. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






