- Submissions

Full Text
Academic Journal of Engineering Studies
Electrochemical Properties of Al2O3-Fe/ Si Composites Prepared by High-Energy Mechanical Milling
Héctor Herrera Hernández*, J Guadalupe Miranda H and C Omar González Morán
Universidad Autónoma del Estado de México, Laboratorio de Investigación en Electroquímica y Corrosión de Materiales Industriales. Blv. Universitario S/N, Predio San Javier Atizapán de Zaragoza, Estado de México 54500, México.
*Corresponding author:Héctor Herrera Hernández DR.3H, Laboratorio de Investigación en Electroquímica y Corrosión de Materiales Industriales, México
Submission: December 09, 2019;Published: January 17, 2020
.jpg)
ISSN:2694-4421 Volume1 Issue2
Abstract
The growing demand in the manufacture of advanced materials with desired and unique properties (e.g. high mechanical strength, durability, good corrosion resistance and low cost of maintenance/replacing) is one of reasons to motivate the researchers to pay special attention in Ceramics as high performance materials for industrial applications. This is because conventional materials cannot meet the engineering requirements during their service that is why the need for advanced ceramic materials to achieve these industrial requirements. In the present work a study was made on Al2O3-matrix ceramic composites reinforced with 2%wt. or 5% wt. of Fe/Si particulates that were produced using a mechanical ball milling at high-energy condition. The electrochemical behavior of these ceramics was investigated by anodic polarization and Electrochemical Impedance Spectroscopy (EIS) measurements in a solution containing 0.5N NaCl, whereas the morphology and microstructural features were examined by optical or Scanning Electron Microscopy (SEM).
Introduction
High Energy Mechanical Milling (HEMM) is a process in which a suitable powder charge (typically, a blend elemental) is repeatedly impacted by stainless steel balls in motion, these balls can roll down freely on the entire surface of the container that allows the impact forces acting on the powder particles during the collision events in order to transmit kinetic energy. The transferred energy is enough, so that the constituent powders are plastic deformed continuously, fractured and cold welded by means of grinding media to form a homogenous nanostructure material with the grain sizes ranging up to 100nm, the process can be performed at room temperature [1,2]. In general, the process HEMM can be defined as the mechanical breakdown of solids into smaller particles without changing the nature of their state of aggregation. This is one of the benefits that mechanical alloying is considered a rapidly developing technology with great industrial interest that can produce a wide range of dispersion strengthened, energetic nanocrystalline, ceramics, metal matrix composites, and other advanced materials [3-5].
Al/Al2O3 matrix is the most popular for MMCs due to its low density, good corrosion resistance and high thermal & electrical conductivity, which has been attractive for use in automobile applications as brake rotor and various components in internal combustion engines [6]. Although the incorporation of reinforcement particles into a metallic or ceramic matrix can enhance the physical and mechanical properties of that material, it can also significantly change the corrosion behavior. The mechanical properties of composites have been investigated in detail [7-10], but more information about their corrosion behavior is also needed. The aim of the present work is providing more information about the electrochemical & corrosion behavior of a reinforced ceramic composite (Al2O3-Fe/Si) produced by high energy mechanical milling. This ceramic composite can be used in heavy duty applications like railway wagon wheels.
Experimental Procedure
Materials & preparation
Al2O3 powder (99.9% purity, 1μm) and Fe/Si powder as that used in steel production, having ∼75wt% Si and ∼23wt% Fe, were used as starting materials. Stoichiometric amounts of the powders were weighed and mixed to achieve compositions of Al2O3-2wt% Fe/Si or Al2O3-wt.5%Fe/Si. The powder mixtures were prepared using a high energy planetary ball mill. The milling was carried out at room temperature in a suitable chromium steel container with ZrO2 balls as grinding elements. The total milling time employed was 4h with a milling speed of 200rpm. Afterward, the mixed powders were subjected to uniaxial cold compaction using a hydraulic press under a constant pressure of 200 MPa on a D2 steel die. Green compacts were sintered at the temperature of 1500 oC during one hour in air atmosphere; the heating rate was 10 oC/min.
Measurements
Morphological and microstructural features were observed using an optical or Scanning Electron Microscopy (SEM). The bulk density of the compacts was determined by the water displacement method using Archimedes’ method. Microhardness was measured on a Vickers Hardness Tester using a diamond indenter. Furthermore, electrochemical measurements in a conventional three-electrode cell were also carried out. Anodic current-potential curves were recorded using a VersaSTAT-4 from -1400 to +400 mV/ EOCP with a scanning rate of 5mV/s. EIS data were collected at EOCP with an IM6-Zahner workstation in the frequency range of 8MHz to 2mHz, with a voltage perturbation of 10mV. Finally, EIS spectra were analyzed by using an appropriate equivalent circuit.
Result and Discussions
The microstructural details of the ceramic composites are illustrated in Figure 1. The micrograph at 10X magnification reveals the formation of highly dense Al2O3-Fe/Si composite, 2.7g/cm3 for Al2O3-2Fe/Si and 2.3g/cm3 for Al2O3-5Fe/Si. However, the dense phase has negligible amount of residual porosity. It also shown in the micrographs of Figure 1 (a,c) two types of phases, a darkgray phase corresponds to the aluminum oxide, whereas the bright particles is the reinforcement material, that could be related to iron aluminate compounds and ferrosilicon. The sharpness surface morphology of the agglomerated particles indicates that during the sintered treatment, a continuous oxide layer grows on the entire particle’s surface due to adsorption of oxygen molecules and moisture from the operating atmosphere. The results also indicate that by increasing the strengthened particles in the ceramic-matrix, the hardness increases from 219 to 492 HV, while the surface roughness of the particles is more notable due to the presence of a thicker aluminum oxide layer.
Figure 1:SEM and optical micrographs at 10X magnifications of the ceramic composites sintered at 1500 oC for 4h in air: (a, b) Al2O3-2Fe/Si y (c,d) Al2O3-5Fe/Si.
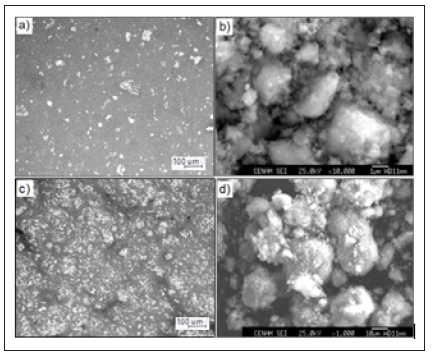
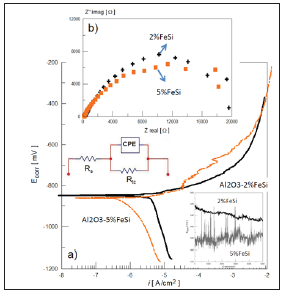
This oxide layer is detrimental to the physical and microstructural properties of the composite, which causes internal microcracks and interfacial debonding between the reinforcement particles (Fe/Si) and the ceramic-matrix due to residual forces in the structure, Figure 1 (c,d). Figure 2 shows the electrochemical spectra for the composite Al2O3-Fe/Si exposed to NaCl solution. In both ceramics a single capacitive loop was observed in the entire frequency range, which is associated to electron charge transfer. The EIS spectra are well analyzed by using the electrical circuit model as given in Figure 2. The value of Rct was ∼19.6KΩ cm2 and Cdl was about 2.7μF/cm2 for Al2O3-2Fe/Si and 81μF/cm2 for Al2O3- 5Fe/Si. EIS response agrees with the anodic curves in which the corrosion current density (icorr) decrease from 10-5 to 10-6A/cm2 (ceramic composite Al2O3-5Fe/Si) indicating that the corrosion reaction is controlled by presence an oxide layer.
Conclusion
Advanced ceramic composites can be successfully produced by high energy mechanical milling process in which the incorporation of fine reinforcement particles can modify the mechanical, physical & microstructural properties, and also may cause substantial changes in the electrochemical & corrosion behavior that can be used in rail way wagon wheels.
Acknowledgement
Authors thank CONACyT for SNI distinction as national researcher. Hector Herrera Hernandez known as DR.3H also would like to thank Sistemas Automatizados e Industriales SA de CV for its materials and equipment support.
Figure 2:Electrochemical measurements for the ceramic composites Al2O3-Fe/Si in 0.5N NaCl: (a) Anodic polarization and b) EIS.
References
- Prasad Yadav T, Manohar Yadav R, Pratap Singh D (2012) Mechanical milling: A top down approach for the synthesis of nanomaterials and nanocomposites. Nanoscience and Nanotechnology 2(3): 22-48.
- Ward TS, Chen W, Schoenitz M, Dave RN, Dreizin EL (2005) Numerical simulation of mechanical alloying in a shaker mill by discrete element method. Acta Materialia 53: 2909-2918.
- Refugio GJG, Miranda HJA, Rodriguez GE, Rocha R (2011) Journal of Ceramic Processing Research 12(3): 319-321.
- Ramezani M, Neitzert T (2002) Mechanical milling of aluminum powder using planetary ball milling process. Journal of Achievements in Materials and Manufacturing Engineering 55(2): 790-798.
- Zhang DL, Raynova S, Koch CC, Scattergood RO, Yousseft KM (2005) Consolidation of a Cu-2.5 vol.% Al2O3 powder using high energy mechanical milling. Materials Science and Engineering 410-411: 375-380.
- Mazaheri Y, Karimzadeh F, Enayati MH (2010) Nanoindentation study of AL356-Al2O3 nanocomposite prepared by ball milling. Materials Sciences and Applications 1(4): 217-222.
- Dai SL, Delplanque JP, Lavernia EJ (1998) Microstructural characteristics of 5083 Al alloys processed by reactive spray deposition for net-shape manufacturing. Metall Trans 29(10): 2597-2611.
- Zhang J, Gungor MN, Lavernia EJ (1993) The effect of porosity on the microstructural damping response of 6061 aluminium alloy. J Mater Sci 28: 1515-1524.
- Miracle DB (2005) Metal matrix composites from science to technological significance. Compos Sci Technol 65(15-16): 2526-2540.
- Reddy BSB, Rajasekhar K, Venu M, Dilip JJS, Das Siddhartha, et al. (2008) Mechanical activation-assisted solid-state combustion synthesis of in situ aluminum matrix hybrid (Al3Ni/Al2O3) nanocomposites. Alloys Compd 465(1-2): 97-105.
© 2020 Héctor Herrera Hernández. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






