- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Research Progresses on Analysis Methods for the Component in Composite Coagulant AS/PDMDAAC
Hao Wang, Xinjie Li and Yuejun Zhang*
School of Chemistry and Chemical Engineering, Nanjing University of Science & Technology, China
*Corresponding author:Yuejun Zhang, School of Chemistry and Chemical Engineering, Nanjing University of Science & Technology, Nanjing, Jiangsu, China
Submission: June 20, 2024; Published: July 02, 2024

ISSN 2578-0271 Volume10 Issue2
Abstract
To investigate the qualitative and quantitative analysis methods for the components in aluminum sulfate/poly dimethyl diallyl ammonium chloride (AS/PDMDAAC) composite coagulant, the existed analytical methods for analyzing independent component AS and PDMDAAC in literatures were reviewed and a three-principles to select appropriate methods from them for the components in the composite was proposed. Based on these principles, suitable matched methods were identified and then ranked according to their practicality. A preliminary feasibility analysis was performed on Fourier transform infrared spectroscopy (FTIR), which is recommended as the preferred analytical method.
Keywords:AS; PDMDAAC; Coagulant; Composite; Component; FTIR; Analysis; Methods
Abbreviations:AS: Aluminum Sulfate; PDMDAAC: Poly Dimethyl Diallyl Ammonium Chloride; FTIR: Fourier Transform Infrared Spectroscopy; AAS: Atomic Absorption Spectroscopy; IC: Ion Chromatography; ICP-MS: Inductively Coupled Plasma Mass Spectrometry; PAC: Poly Aluminum Chloride; NaTPB: Sodium Tetraphenylboron; CTMAB: Cetyltrimethylammonium Bromide; HBP-QAT: Hyperbranched Polymer with Quaternary Ammonium Terminal; PVSK: Potassium Vinyl Sulfonate; TBO: Toluidine Blue O; ATR: Attenuated Total Reflectance
Introduction
Aluminum sulfate/poly dimethyl diallyl ammonium chloride (AS/PDMDAAC) is a new type of composite coagulants. The varying proportions of its components make it suitable for broad applications in treating various surface water and wastewater [1]. Specifically, it is an efficient flocculant and decolorizing agent for printing and dyeing wastewater in textile industry [2]. As this new agent continues to be promoted and applied, there is an urgent need to establish corresponding industry and national quality standards. However, the foundation for these standards lies in the qualitative and quantitative analysis methods of the components in the composite. Until now, unfortunately, no direct method for analyzing the two components, AS and PDMDAAC, in the composite has been reported due to mutual interference between the components and difficulties in effective separation. Therefore, based on the summarization of existing analysis methods for the components and the authors’ experience, a proposal was set up in this paper to research on an approach to establish qualitative and quantitative analysis methods for the components of the AS/PDMDAAC composite coagulant, along with a preliminary feasibility analysis.
Qualitative and quantitative analysis methods for AS
The detection methods for AS, in generally can be categorized into chemical analysis and instrumental analysis methods, both of which get the amount of AS by measuring the content of Al3+ or SO42-. A commonly used chemical analysis method is the EDTA titration of aluminum ions (Al3+), followed by metal ion back-titration to measure the Al3+ content, which is then converted to the AS content (expressed as aluminum oxide Al2O3). This is a direct quantitative analysis method. According to the Chinese national standard GB/T 31060-2014 [3], this method is applicable for measuring aluminum oxide content in the range of ω(Al2O3)≥ 0.01%. Although this method provides direct measurement of the sample, the preliminary preparation takes up to 3 h·(person · sample)-1, and the titration analysis takes about 10 min·(person · sample)-1. During the analysis, other cations in the AS sample, such as Fe3+, can also react with EDTA, which may affect the accuracy of the results.
The gravimetric method using BaSO4 is an indirect quantitative analysis method. It involves adding a standard solution containing Ba2+ to the testing sample solution containing SO42-. The resulting BaSO4 precipitate is weighed to determine the SO42- content, which is then converted to the AS content. The method has a wide testable range [ρ(SO42-)=(10~5000)mg/L] [4]. Although this method directly measures the SO42-) in a sample, the precipitate needs to be dried to a constant weight, so that the operation time- takes up to 4 h·(person · sample)-1. During the titration analysis, other ions that can precipitate with barium salts, such as CO32- and SO3 2-, may interfere with the results, affecting the measurement accuracy.
In instrumental analysis methods, Fourier transform infrared spectroscopy (FTIR) is based on the characteristic absorption peaks and their strength of bonds vibrations and rotations in a molecule at specific infrared wavelengths. It is used for both qualitative and quantitative analysis. Wei et al. [5] used the FTIR method to qualitatively analyze the Na2SO4 solution, confirming the presence of νas(SO42-) and its concentration by the characteristic peak at 1133cm-1 and its peak area. This method has a wide range for determining SO42- [ω(AS)≥0.1%] [6]. The preliminary preparations, such as creating the standard curve, take about 2 h·(person · batch)-1, while the measurement itself takes about 2~5 min·(person · sample)-1. Other substances that produce the peaks at the same peak position can interfere with the qualitative and quantitative analysis results.
Atomic absorption spectroscopy (AAS) is based on the absorption of characteristic spectral lines produced by ground-state metallic elements in the vapor of the testing sample, which excites them and allows for their direct qualitative and quantitative analysis in the sample. According to the Chinese National Standard GB/T 23837-2009 [7], the sample is acidified with nitric acid and digested to extract aluminum atoms in the water sample. This extracted solution is then aspirated into the AAS flame, and its absorbance at a wavelength of 309.3nm is measured to determine the aluminum content. The method has a testable range of ρ(Al)=(5~100)mg/L. The preliminary preparations, such as creating the standard curve, take about 2 h·(person · batch)-1, while the analysis operation itself takes about 1~10 min·(person · sample)-1. The aluminum content analysis results can be converted to AS content, but it is important to note that the total salt content in the sample solution should not exceed 15g/L, as higher salt concentrations can interfere with the results.
Spectrophotometry is a method based on the selective absorption of light by the analyte in a testing solution, combined with the Lambert-Beer law, and it uses a standard curve for quantitative analysis. The Chinese National Standard GB/T 23944- 2009 [8] employs this method to determine the Al3+ content in samples, with a testable range of ρ(Al3+)=(0.02~0.2)μg/mL. When analyzing AS, its content can be calculated based on the Al3+ content. The sample preparation and analysis procedures for this method take about 1.5 h·(person · batch)-1. Additionally, the chromogenic reagent Chrome Azurol S can form complexes with other metal ions such as Fe3+, which can interfere with the determination of Al3+ content at the same absorption wavelength.
Ion chromatography (IC) is a method that separates ions in the mobile phase by exchanging them with ions on a stationary phase ion-exchange resin. It uses chromatographic retention time and peak area-content standard curves for qualitative and quantitative analysis. This method can determine the SO42- content, which can then be converted to the AS content. The Chinese National Standard GB/T 14642-2009 [9] uses this method to determine the SO42- content in water samples, with a preparation time of about 2 h·(person · batch)-1 and an analysis time consumed of 3~10 min·(person · sample)-1. The method has a testable range of ρ(SO4 2-)=(0.20~500.0)mg/L. High concentrations of other anions in the solution can cause interference, requiring pretreatment to remove them or dilution of the sample.
Potentiometric titration involves inserting a reference electrode and an indicator electrode into the sample solution to quantitatively analyze the ions by measuring the relationship between the electromotive force of the cell and the related ion activity in the sample. The Chinese National Standard GB/T 6911-2017 [10] uses this method with a Ba2+ ion-selective electrode and a reference electrode, titrating SO42- in water samples with a BaCl2 standard solution. The analysis results can be converted to the AS content. Similarly, this method does not require a standard curve and has a measurable range of ρ(SO42-)=(5~1000)mg/L. The preparation time is only 1 h·(person · sample)-1, and the analysis time is 5 min·(person · sample)-1. However, if phosphates or polyphosphates are present in the solution, they will cause significant interference and need to be removed by dilution or co-precipitation.
Inductively coupled plasma mass spectrometry (ICP-MS) analysis is based on ionizing the sample at high temperatures, followed by separating and detecting the ion signals and their masses with a mass spectrometer for qualitative and quantitative analysis. Nong et al. [11] used ICP-MS to analyze Al3+ in the water treatment agent poly aluminum chloride (PAC), converting it to PAC content based on Al3+ levels. The method has a measurable range of ρ(Al3+)=(1.0~2.0×106)μg/L. However, if this method is used to analyze AS, the AS content can be calculated from the Al3+ analysis results. The method requires approximately 3 h·(person · batch)-1 for sample pretreatment by nitric acid decomposition and preparation of standard solutions for instrument calibration, while the analysis time is only 2~5 min·(person · sample)-1. The minor plasma interferences produced by ICP-MS (e.g., the interference of 27Al+ with 54Fe2+) can be removed by increasing the mass spectrometer resolution [12] or using collision/reaction cell technology [13].
Qualitative and quantitative analysis methods for PDMDAAC
PDMDAAC belongs to a type of quaternary ammonium salts. Its qualitative and quantitative detection methods can also be divided into chemical analysis and instrumental analysis methods, and can be conducted by analyzing its molecules, quaternary ammonium cations, or Cl-. In the chemical analysis method, the sodium tetraphenylborate (NaTPB) titration-cetyltrimethylammonium bromide (CTMAB) back titration method is a direct quantitative analysis method for determining the content of quaternary ammonium cations, i.e., the number of quaternary ammonium units. Li et al. [14] accurately determined the content of several quaternary ammonium salts using this method, with a detection limit of ω(quaternary ammonium salt)≥0.10%. The method requires approximately 1 h·(person · sample)-1 for preliminary preparation and 15 min·(person · sample)-1 for analysis. Additionally, this method is suitable for analyzing the cationic unit fraction-the cationicity-in poly quaternary ammonium salts. For example, Feng et al. [15] used this method to measure the cationicity of hyperbranched polymer with quaternary ammonium terminal (HBP-QAT) as 44.2%. However, this method determines the total amount of all types of quaternary ammonium units in the solution, rather than the content of a single quaternary ammonium salt.
The potassium polyvinyl sulfate (PVSK) colloidal titration method is a quantitative analysis method for determining the cationicity in poly quaternary ammonium salts. This method uses toluidine blue O (TBO) as an indicator and performs precipitation titration with a standard solution of low-molecular-weight anionic polymer PVSK. By calculating the anion content of the PVSK required for titration, the cationicity of the poly quaternary ammonium salt can be determined. Mocchiutti et al. [16] accurately determined the content of PDMDAAC using this method. The preliminary preparation for this method takes approximately 5 h·(person · batch)-1, and the titration process takes 0.5 h·(person · sample)-1. However, when the polymer concentration in the solution is high, the resulting colloidal precipitate may adsorb the indicator, making it difficult to determine the endpoint [17]. Therefore, selecting the titration concentration based on the sample’s molecular weight is particularly important.
The Mohr method uses K2CrO4 as an indicator and AgNO3 standard solution to perform precipitation titration of Cl- in water samples. The results of Cl- analysis can be indirectly used to calculate the cationic unit content of PDMDAAC. According to the Chinese National Standard GB/T 15453-2018 [18], the Mohr method is used to determine Cl- concentrations in water samples within the testable range of ρ(Cl-)=(3~150)mg/L. The preliminary preparation for this method takes approximately 4 h·(person · batch)-1, and the titration analysis takes about 20 min·(person · sample)-1. This method is susceptible to interference from other ions that form precipitates with AgNO3, such as SO42-.
In instrumental analysis method, the FTIR method can be also used as a basis for both qualitative and quantitative analysis of the PDMDAAC quaternary ammonium salt functional group by identifying the characteristic absorption peak of δas(N-CH3) around 1470cm-1 and its strength in the sample. The testing range for this method is ω(PDM)≥0.1%. Levakov et al. [19] used FTIR for the qualitative analysis of PDMDAAC. By employing the attenuated total reflectance (ATR) technique, they avoided the drawback of accurately adding amount of the sample, which is a necessity of the traditional KBr pellet method, originally. The preliminary preparation for this method takes approximately 1 h·(person · batch)-1, and the analysis operation time can be shortened to 2 min·(person · sample)-1. Similarly, the presence of other substances with functional groups that exhibit peaks at the same wavenumber can interfere with the qualitative and quantitative analysis results.
Spectrophotometry can also be used for the direct quantitative analysis of quaternary ammonium salts. Liu et al. [20] used a spectrophotometer at 500nm to measure the formation of a pink ion-association complex between titan yellow and CTMAB under alkaline conditions. The measurable range of this method is approximately ρ(quaternary ammonium salt)=(0~12)mg/L. Other cations in the solution, such as Al3+ and Fe3+, can directly participate in the association, thereby interfering with the analysis.
Due to the difficulty of analyzing polymeric substances with IC, necessary pre-treatment steps such as precipitation and alkaline fusion are required to separate or degrade and remove them. Therefore, when analyzing PDMDAAC, NaTPB can be added to precipitate and separate the quaternary ammonium cations. Subsequently, the residue Cl- are qualitatively and quantitatively analyzed based on the chromatographic retention time and peak area. The results are then converted to the PDMDAAC content through the cationic unit contents. According to the Chinese national standard GB/T 14642-2009 [9], this method determines Cl- concentration in the testable range of ρ(Cl-)=(0.10~500)mg/L. The preliminary preparation of this method takes approximately 5 h·(person · batch)-1, and the chromatographic analysis time is about 5 min·(person · sample)-1. Other high-concentration anions, such as SO42-, can interfere with the results, and thus need to be removed or diluted in advance.
Potentiometric titration is also a simple method for quantitatively determining Cl- content, and based on the results, it can be used for the indirect quantitative analysis of PDMDAAC. According to the Chinese national standard GB/T 15453-2018 [18], an Ag/AgCl electrode is used as the reference electrode and an Ag electrode as the indicator electrode, with the AgNO3 standard solution being automatically titrated to the endpoint determined by potential. The testable range of this method is ρ(Cl-)=(5~1000) mg/L. The presence of SO42- during titration significantly interferes with the results.
Additionally, ICP-MS can also be used to analyze Cl-. Nie et al. [21] precisely determined the chlorine content in dust-free samples using this method after acidifying and digesting the samples with ultrapure nitric acid. Similarly, the PDMDAAC content can be quantitatively analyzed based on the results of Cl- analysis. The detection limit of this method can reach ρ(Cl-)≥0.06μg/L.
Suggestions for qualitative and quantitative analysis methods of AS/PDMDAAC
A three-principles for screening research methods and
their efficiency ranking: From the literature summarized
in Sections 1 and 2, it is known that the two components, AS
and PDMDAAC in the composite coagulant, each has its own
independent and mature qualitative and quantitative analysis
methods. These methods can serve as the basis for analyzing the
components in the composite. However, the uniform dispersion of
the two components due to their mutual solubility and the wide
relative content distribution poses challenges. The former makes
the approach of simple separation followed by analysis unfeasible,
while the latter restricts the detection limit to the presence of
extremely low content of either component in the composite.
Therefore, to develop a method suitable for further research that
directly uses the composite coagulant as the sample and features
rapid, accurate, and low-cost analysis, this paper proposed the
screening of AS/PDMDAAC component analysis methods based on a
three-principles derived from single component analysis methods.
The methods to be studied should meet the following criteria:
A. When detecting any component in the composite, it should
not be interfered with other component, or such interference
should be measurable.
B. The detection limits (testable range) for each component in
the composite must be closer or similar.
C. The method should be able to measure both components in
the composite, parallel or simultaneously. Additionally, the
selected methods should be ranked based on their rapidity in
speed (time consumption) and ease of industrial application
(low cost).
Screening results and ranking of analysis methods:
The analysis methods from Sections 1 and 2 that meet all three
principles, along with their operational characteristics, time and
cost consumption, on one side, and uncertainty factors on the
other side, are summarized in Table 1. In this paper, the adjustable
range of the mass ratio of the two components in the AS/PDMDAAC
composite coagulant [ω(PDMDAAC):ω(AS)] is preliminarily
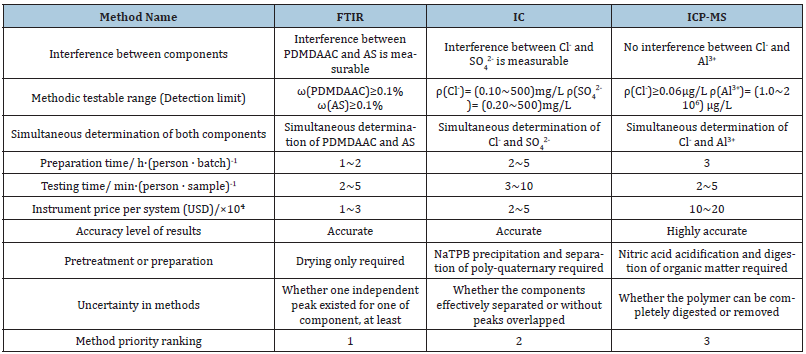
supposed to be 1:50 to 50:1. From Table 1, it can be seen that:
firstly, FTIR, IC, and ICP-MS methods all meet the requirements of
all three principles. Secondly, each of the three methods has its own
uncertainties for further analysis, namely:
a) In FTIR, whether the composite’s spectrum contains a distinct
absorption peak for one of two, at least or not;
b) In IC, whether the components can be effectively separated or
if their chromatographic peaks will be not overlapped;
c) In ICP-MS, whether the pre-treatment process can completely
digest the polymer or not. Finally, considering procedure
speed, accuracy, and cost, the priority order of the three
methods from high to low is: (1) FTIR, (2) IC, (3) ICP-MS.
Table 1:Analysis methods and specific criteria which fully satisfy the three principles.
Table Abbreviations: Fourier transform infrared spectroscopy (FTIR); Ion chromatography (IC); Inductively coupled
plasma mass spectrometry (ICP-MS); Poly dimethyl diallyl ammonium chloride (PDMDAAC); Aluminum sulfate (AS);
Sodium tetraphenylborate (NaTPB).
FTIR spectrum analysis results: Based on the work done in Section 3.2, the method with the highest priority is the FTIR method. Here, ATR-FTIR method was used to measure solid samples of AS, PDMDAAC, and AS/PDMDAAC [ω(PDMDAAC):ω(AS)=1:1], in order to observe whether the composite component spectrum contains distinct characteristic absorption peaks, one of which is independently, at least or not, so that the preliminary feasibility of using the FTIR method to test the components, qualitatively and quantitatively in AS/PDMDAAC, could be confirmed. The obtained spectra are shown in Figure 1. From the spectrum of composite in Figure 1 and in comparison of all three spectra with each other, it can be seen that in the FTIR spectrum of AS/PDMDAAC, the peak at 3047cm-1 is mainly formed by the overlapping of the ν(-OH) of water [22] and the ν(Al-OH) of AS [23], which covers the original PDMDAAC absorption peaks at 3003cm-1 [νas(-CH3)] [24], 2931cm- 1[νas(-CH2)], and 2867cm-1 [νs(-CH2)] [25] in this range. The peak at 1646cm-1 is attributed to the δ(-OH) of water [22]. The peak at 1471cm-1 is an independent absorption peak of PDMDAAC’s δas(NCH 3) [24]. The peak at 1041cm-1 is a composite peak formed by AS’s νas(SO42-) [26] and other peaks of PDMDAAC within a certain wavenumber range, such as 1305cm-1, 1244cm-1, and 1138cm-1 [ν(C-N)] [27], as well as 947cm-1 [γ(-CH)] [22]. The peak at 593cm-1 is formed by the overlapping of AS’s δas(SO42-) [28] and δ(Al-OH) [29].
Figure 1:FTIR spectrum of samples of AS, PDMDAAC, and AS/PDMDAAC [ω(PDMDAAC): ω(AS)=1:1].
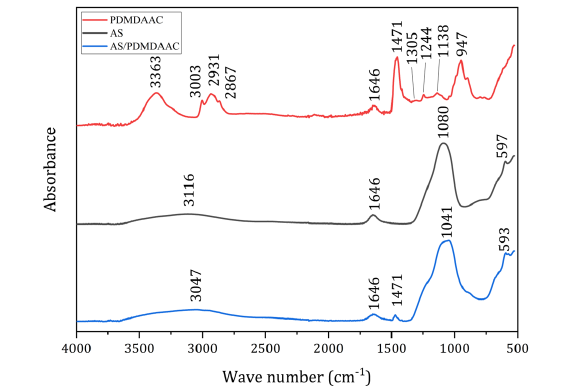
In the FTIR spectrum of the AS/PDMDAAC composite, since the solid sample still contains a small amount of water, the absorption peaks associated with water are not considered in this use. Although the peak at 593cm-1 is an independent absorption peak of AS, its peak shape is poorly symmetrical, making it probably difficult to use as a quantitatively testable peak. The other independent (or minimally interfered) absorption peaks are located at 1471cm-1 and 1041cm-1. The former can be used as a quantitatively testable peak for characterizing PDMDAAC, but the position and shape of the latter peak may vary with the relative content of AS and PDMDAAC, leading to multiple overlapping phenomena in the spectra of the two components in the composite. This might provide fortunately the possibility for subsequent research on calculating their mutual change of the two component contents in the composite. Thus, the preliminary feasibility of research on the FTIR method for the qualitative and quantitative analysis of two components in composite AS/PDMDAAC was verified.
Conclusion
For the new type of composite coagulant AS/PDMDAAC with its two components, in order to establish a qualitative and quantitative analysis method of the rapidity, accuracy, and easy to apply, the analytic principles, detection limits, and anti-interference characteristics of existing analysis methods for AS and PDMDAAC were reviewed in this paper. Then three principles of screening the methods for the component analysis in the composite coagulant were proposed, the FTIR, IC, and ICP-MS methods were selected accordingly and ranked by their priority in applicability. Finally, a preliminary feasibility on the FTIR method recommended for further research was verified.
Conflict of Interest
No conflict of interest.
References
- Adebayo I O, Olukowi O O, Zhiyuan Z, Zhang Y (2021) Comparisons of coagulation efficiency of conventional aluminium sulfate and enhanced composite aluminium sulfate/polydimethyldiallylammonium chloride coagulants coupled with rapid sand filtration. Journal of Water Process Engineering 44: 102322.
- Zaharia C, Musteret CP, Afrasinei MA (2024) The use of coagulation–flocculation for industrial colored wastewater treatment-(I) The application of hybrid materials. Applied Sciences 14(5): 2184.
- GB/T 31060 (2014) Water treatment chemicals-Aluminum sulfate.
- Ma Y, Ma Z, Zhang X (2017) Comparison of water soluble SO42- chemical analysis methods in soil. Chenmical Interediate (7): 21-22.
- Wei X L, Gao M G, Liu J G, Xu L (2013) Quantification of sulphate in ambient aerosols by FTIR spectroscopy. Advanced Materials Research 718-720: 1136-1139.
- Weng S, Xu Y (2016) Fourier transform infrared spectroscopy analysis. (3rd edn), Chemical Industry Press, Beijing, China, pp. 1-508.
- GB/T 23837 (2009) Industrial circulating cooling water-Determination of aluminium-Atomic absorption spectrometric methods.
- GB/T 23944 (2009) Inorganic chemicals for products use-General method for the determination of aluminium-Chromazurol S-spectrophotometric method.
- GB/T 14642 (2009) Industrial circulating cooling water and boiler water-Determination of fluoride, chloride, phosphate, nitrite, nitrate and sulfate-Ion chromatography.
- GB/T 6911 (2017) Determination of sulfate in water for industrial circulating cooling system and boiler.
- Nong J, Lu Y, Zhu R (2000) The determination of Al_2O_3 and 5 elements in poly aluminium chloride by inductively coupled plasma mass spectrometer method. Industrial Water Treatment (2): 37-39.
- Su D, Liang B, Luo T, Zhang J, Chen Y, et al. (2021) The determination of aluminum content in 89SrCl2 crude material by HR-ICP-MS. Journal of Isotopes 34(5): 468-474.
- Zhou X, Liu H (2018) Accurate determination of calcium and chlorine in food with inductively coupled plasma tandem mass spectrometry. Spectroscopy and Spectral Analysis 38(11): 3567-3571.
- Li Y, Xie J, Qu C, Wang YW (2009) Determination of quaternary ammonium using back titration analysis of sodium tetraphenyl boron. Journal of Xi'an Shiyou University (Natural Science Edition) 24(4): 62-64.
- Feng Q, Liu H, Peng Z, Zheng Y (2020) Synthesis, characterization and evaluation of long-acting hyperbranched cationic polymer clay stabilizer used in water flooding. Polymer Testing 82: 106344.
- Mocchiutti P, Zanuttini M (2005) A useful equation for estimating the surface charge of pulp fibers. Tappi Journal 4(5): 18-22.
- Gu X, Tian S, Zhang Y (2003) Determination of cationic monomer conversion in copolymer PDA. Speciality Petrochemicals (6): 15-18.
- GB/T 15453 (2018) Determination of chloride in water for industrial circulating cooling system and boiler.
- Levakov I, Maor I, Barak C, Kirshenbaum Y, Rytwo G (2023) Colorimetric quantification for residual Poly-DADMAC in water treatment. Water 15(19): 3352.
- Liu Y, Ma W, Zhou L, Peng F (2010) Spectrophotometric determination of cetyltrimethylammonium bromide in water with titan yellow. China Surfactant Detergent & Cosmetics 40(3): 221-224.
- Nie L, Ji H (2006) Determination of trace chloride in cleaning products by ICP-MS. Guangdong Trace Elements Science (6): 48-52.
- Yu H, Gao B, Shao X, Yue Q, Zhao H, et al. (2001) Study on the dimethyldiallyammonium chloride by infrared spectrum. Journal of Shandong University (Natural Science) (3): 330-335.
- Lal K, Garg A (2017) Physico-chemical treatment of pulping effluent: Characterization of flocs and sludge generated after treatment. Separation Science and Technology 52(9): 1583-1593.
- Guo Y, Li X, Sun J, Liu Y, Wang H, et al. (2022) Physicochemical characterization and flocculation performance evaluation of PAC/PMAPTAC composite flocculant. Journal of Applied Polymer Science 139(7): 51653.
- Zhang Y J, Jia X (2016) Synthesis of ultra-high molecular weight poly (dimethyldiallyl ammonium chloride). Russian Journal of Applied Chemistry 89(2): 315-323.
- Wang YS, Lim S, Wang XY (2023) Evaluation of the early strength of limestone-slag-cement ternary composite mortar improved by aluminum sulfate. Journal of Materials Research and Technology 26: 2601-2616.
- Xu Q, Peng J, Zhang W, Wang X, Lou T (2020) Electrospun cellulose acetate/P(DMDAAC-AM) nanofibrous membranes for dye adsorption. Journal of Applied Polymer Science 137(15): 48565.
- Refat MS, Elsabawy KM (2010) Synthesis, spectral, thermal, structural and micro-structural features of new nano-(Zn, Al and Bi) compounds. Journal of Molecular Structure 984(1-3): 287-293.
- Sun T, Sun CH, Zhu Gl, Miao Xj, Wu CC, et al. (2011) Preparation and coagulation performance of poly-ferric-aluminum-silicate-sulfate from fly ash. Desalination 268(1-3): 270-275.
© 2024 Yuejun Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






