- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Survismetry for Survistronics Enlarging Potential of Surfaces for Industrial Applications
Man Singh1*, Vijay Kumar Srivastava2, Sunita Singh3 and Ch Ramakrishna4
1School of Chemical Sciences, Central University of Gujarat, Gandhinagar 382030, Gujarat, India
2Vice Chancelor, Maharaja Sayajirao University of Baroda, Gujarat, India
3Department of Biochemistry, Shivaji College, University of Delhi, India
4Department of Environmental Studies, GITAM University, Visakhapatnam-530 045, India
*Corresponding author:Man Singh, School of Chemical Sciences, Central University of Gujarat, Gandhinagar 382030, Gujarat, India.
Submission: December 05, 2022; Published: December 09, 2022

ISSN 2578-0271 Volume 7 Issue4
Opinion
Friccohesity chemistry and physicochemical states of the soft or hard surfaces have become academically as well as industrially the hub of ideas and web of interdisciplinary and transdisciplinary research leading to substantially boost up the overall attraction towards photoluminescence, refraction, diffraction, and reflection and polarized lights. The optronics, electrotonic, nano thin films, coatings, polishing, dyeing, encapsulation, and various others have been the friccohesity of the unpaired electrons, π-conjugation, and electrostatic dipolar alignments where the friccohesity resonating energy transfer to either neighbour atom of a specific wavevector or space as emitted photon or phonons generate the valuable-coloured light. Such colour science or coloronics has been emerged out a potential or cohesive forces of the atoms with specific electronic configuration which generate the single valued wavefunction. The electrons of these atoms rotate, vibrate to translate from one point to another and many times these electronic motions also vibrate the atoms of the molecules which adhere to another atom of other molecules generating different response of the sunlight or any other light
The nano emulsions are the popular examples where the dipoles of the water adhere to the surfactants, oil, or other molecules out of their cohesive forces. The pattern of the adherence generates out to the degeneracy of the energy levels of the electron’s response to the light else no colour could be generated. The argon, krypton or any other gases do not have single or unpaired electron and are unable to generate photoluminescence on interacting with sunlight. The surface energy of ideal gases is almost zero while the cohesive energy is almost the 100%. Thus, the surface energy depicts the surface electronics while the cohesive bulk or a rheology as a shear stress with specific viscous electronic motions engineer an interfacial tension (IFT,mN/m). The IFT is a function of surface electronics and viscous electronics is measured with survismeter and is noted as survistronics expressed as a friccohesity chemistry depicting specific colour emitted by the particular surface of photosensitive and photoluminescent materials like dyes, inks, and light splitting surface. The friccohesity as a measure of IFT is noted as survismetry and friccohedics. These tactics elaborate the shear stress and rate, surface energy, and mutual interaction coefficient of photoluminescent molecules with monodispersing solvent. Survismetry deals with to integrate the surface tension, interfacial tension, wettability, viscosity, friccohesity, nanoparticle surface area and size including a coagulability of nano emulsion interfaced through the hydrophobicity and hydrophilicity, πi-conjugation activities, induced dipolar activities together by saving 95% laboratory resources, chemicals, and time.
These parameters determine molecular interactions for better industrial material formulation like textile, dyeing, coating, caping, nanothin film forming through the interacting atoms of functional molecules like graphene oxide (GO) with variable potential energy well and tentropy. Our previously reported study about aqueous solutions of GO inferred a bottom up of 2 dimensional (2D) photoluminescent GO sheets through the survistronics. The monodispersed GO sheets were separated as the H2O dipoles were aligned around the individual sheets making a continuous soft nanothin film through the dipolar interactions developing a nanohydration sphere (NHS). Therefore, the 2D GO sheets remained homogeneously suspended within the whole solution as a stronger adhesive force generated the stronger frictional forces. On heating the aq-GO solution to 102 ℃ for 30 min, it was kept overnight at NTP which had weakened the adhesive or frictional forces working between the NHS and the GO sheets. On weakening of the frictional forces, the operative chemical linkages of NHS loosened a grip over NHS and as a result the NHS have shifted to a water phase developing the cohesive forces and the individual GO sheets got free to bottom up by coming together on developing the stronger cohesive forces.
This fundamental mechanistic science is the friccohesity chemistry as the chemistry of the water phase and the GO bottom-up sheets depicted different physicochemical properties. The surface tension of the water was increases and the viscosity decreased, these were measured together using survismeter without dislocating or transferring the sample to many devices. This mechanism could be noted as phasing out of the GO sheets from bulk to the surface as the GO sheets are light. However, the heavier particles are phased out to settle at the bottom. Therefore, the survismeter measures leaching out pollutants from a polluted liquid sample to another solvent. Homogeneous, volatile organic compounds (VOC), carcinogenic organic compounds (COC) liquids are jacketed to avoid escape. The survismeter retrieves the chemical linkages and thermodynamic coordinates established among the constituents of the liquid nanoformulations. The survismeter makes a complete physicochemical characterization without subjecting a sample to mechanically, spectro photochemically, and molecular motions and also to external forces like magnetic, thermal, and electrical that may damage structures of the different devices [1]. Use of survismeter minimizes the wastage of chemicals, time, electricity, glass blowing gases, and save environment. The surface tension, interfacial tension, wettability, viscosity, friccohesity, particle surface area and size, coagulability of liquid mixtures, nano emulsion, hydrophobicity and hydrophilicity, π-conjugation activity, induced dipolar activity are used in areas of chemical formulations, pharmaceuticals, cosmetics, oil, and petroleum, dyeing and textile, polymers, pesticides, and others. The favourable or non-favourable shears are similar to an orientation of Fe3+ with 3d5 and Gd3+ with 4f7 electrons as these cations generate magnetic field and magnetic resonance imaging (MRI) contrasting intensities respectively. Contrary to conventional devices, the survismeter has a great provision to optimize the molecules via orient, reorient, and streamlining to express an overall impact on a resultant friccohesity. Each functional group with intrinsic electronic cloud contributes to sum up fluid dynamics, lone of electrons, π-conjugation and spatial geometry, electrostatic forces to estimate topographical entropy noted as tentropy macromolecules like proteins. The contribution of each constituent is summed up as physicochemical property. Pyridine, benzene, cyclohexane, nitrobenzene, aniline, phenol, benzoic acid, benzamide and others could be taken to determine a tentropy. The functional groups reorient electronic cloud of molecule to express variable friccohesity as per structures (Figure 1).
Figure 1:Capacitance-voltage cyclic curves for СН900-20 for 48% H2SO4 at potential rates: 1-0.5mV/s, 2-1mV/s, and 3, 4-2mV/s.
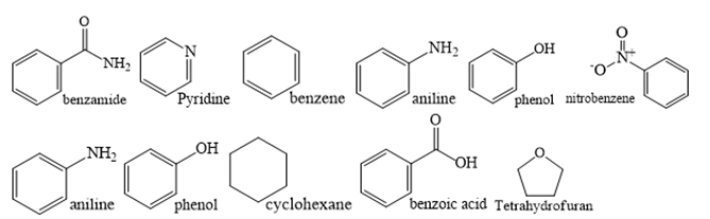
The structural constituents generate multiple localized interacting atoms which induce interacting activities as per their electronic cloud and numbers of multiple sites generating a specific friccohesity expressed as generating the velocity gradient (dv⁄dy) [2,3] (Figure 2).
Figure 2:Illustrates friccohesity model of fluid flow incorporating CF and FF together.
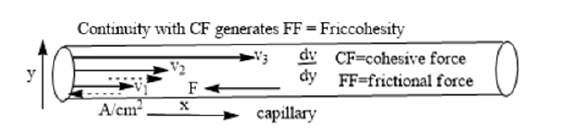
F opposing force applied on unit area A , laminar flow scans a structural orientation with solvent expressed as.
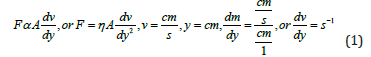
Proportionality constant η is expressed as under which is generated due to the surface charges of a capillary passing a fluid through its fixed internal radii expressed as under.

Stronger opposing forces develop a lowest shear rate and takes a longer viscous flow time due to higher relaxation time in a streamlining capillary (Figure 3).
Figure 3:Higher shear stress and lower shear rate generated due to variable friccohesity.
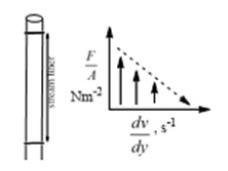
However, the fluid flow, a laminar as Newtonian or non- Newtonian is possible when the molecules constituting a liquid are interconnected with specific cohesive forces forming layer to facilitate flow over an adjacent layer with specific adhesive forces formulating a friccohesity expressed as.
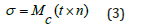
The σ friccohesity, r radius of drop, Mc Man Singh constant, t viscous flow time (vft), n pendant drop number (pdn). The size and numbers of pdn depends on cohesive forces, stronger are the cohesive forces larger is a size and lower are the numbers while a higher adherence increases the vft and lower pdn could be correlated as under.
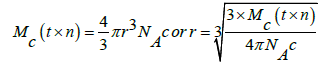
NA Avogadro number, and c is concentration. Thereby the adherence and coherence are the most fundamentals to resonate energy to mutually distribute or transfer for equilibrating and maintaining a stability without generating any specific patch of a surface which could not mutually distribute or transfer the energy. The hydration number of the water molecules which adhere could be determined using the pdn that infers the cohesive and adhesive forces on a liquid-solid and Liquid-liquid interfaces as.
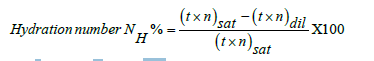
The t is vft, n pdn sat saturated, and dil infers dilute solution respectively. The vft infers adherence of solute particles with solvent on transforming their potential energies into the oscillating through rotational, vibrational, and electronic motions which translate solute and solvent for dispersion. The hydration numbers of solvent directly influence the shape and height of a pdn as the ionic charges which channelize the water dipoles are counterbalanced as per concentration of the solute or electrolyte. Friccohesity prominently elucidates in situ coagulation which affect vft developing a cohesion or cohesive energy or surface energy with higher surface tension [3]. There is an urgent need to determine viscous force as well as anti-viscous force where antviscous forces are coagulating forces. Both the forces are measured with vft and pdn, their product is friccohesity. This is a reason that the molecules during viscous flow may also undergo reorientation, rotational, vibrational, translational motions due to shear forces or rheological changes. It generates a prominent possibility for developing surface energy. Thus, the friccohesity most accurately elaborates structural expression of molecules. Each structure generates specific friccohesity data.
References
- Singapore Govt Patent No. 126089, Novel instrument for measurement of surface tension and viscosity of solutions. Commercialized by Borosil Ltd, catalogue no. 3453 listed as Borosil Mansingh Survismeter.
- Singh M (2006) Survismeter-type 1 and 2 for surface tension and viscosity measurements of liquids for academic, and research and development studies, Journal of Biochemical and Biophysical Methods 67: 151-161.
- Kumar K, Dave RP, Dev S, Singh M (2022) Study of molar properties of GO after doping with transition metals for photodegradation of fluorescent dyes. RSC Adv 12(46): 29734-29756.
© 2022 Man Singh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






