- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Activated Carbon to Improve the Performance of Membrane Processes for the Extraction and Elimination of Methylene Blue Pollutant
Saâd Oukkass1, R Ouchn1,3, I Mechnou1, I El Yaakouby1, L Lebrun2 and M Hlaïbi1,2*
1Materials Engineering for the Environment and Valorization Laboratory (GeMEV), I3MP team, Aïn Chock Faculty of Sciences, B.P. 5366, Maârif, Casablanca, Morocco
2Membranes team, Polymers, Biopolymers, Surfaces Laboratory (PBS), UMR 6522 of the CNRS Faculty of Sciences F-76821 Mont Saint Aignan, France
3Laboratory of Research on Textile Materials (REMTEX), ESITH Casablanca, Morocco
*Corresponding author:M Hlaïbi, Membranes team, Polymers, Biopolymers, Surfaces Laboratory (PBS), UMR 6522 of the CNRS Faculty of Sciences F-76821 Mont Saint Aignan, France
Submission: July 27, 2022; Published: November 09, 2022

ISSN 2578-0271 Volume 7 Issue3
Abstract
In the last few years, membrane technologies have experienced a considerable growth, due to their numerous uses and their advantages over other conventional techniques. The membrane processes present today an important research topic, especially affinity polymer membranes, very adapted to oriented processes. Methylene blue is one of the most widely used dyes in various fields such as chemistry, medicine, dentistry and the dye industry. Our objective is to develop a polymeric inclusion membrane for methylene blue extraction and recovery processes, assisted by activated carbon. We developed a membrane based on a mixed polymer support, polyvinylidene fluoride (PVDF), polyvinylpyrrolidone (PVP), and the amphiphilic molecule Tween 20 (TW20) as extractive agent (EA). In order to quantify the performance of developed membrane, macroscopic parameters such as initial flux J0, and permeability P, as well as microscopic parameters such as association constant Kass, and diffusion apparent coefficient D*, were determined. Then, we determined the activation parameters, energy (Ea), enthalpy (ΔH# ass), andentropy (ΔS#). Finally, we studied the effect of activated carbon on the evolution of processes carried out and the performance of the membrane, and we observe a very clear improvement. The obtained results indicate that the membrane developed for the studied processes has a real potential for the techniques of separation and pre concentration.
Keywords: Methylene blue; Polymer inclusion membrane; Permeability; Extraction
Introduction
Methylene blue or methylthioninium chloride is an organic compound whose name in systematic nomenclature is 3,7-bis-(dimethylamino) phenazathionium. It is the most commonly used dye in the dyeing of cotton, wood and silk [1]. It was first synthesized by Heinrich Caro in 1876. This compound can be prepared by treating dimethyl-4- phenylenediamine with hydrogen sulfide dissolved in hydrochloric acid and then oxidizing it with chloride ferric [2].
It is not highly dangerous but has a harmful effect on living organisms [3] and waters [4]. Acute exposure to this product will cause skin irritation and permanent eye damage, rapid or difficult breathing [4] and increased heart rate by inhalation [5], irritation of the gastrointestinal tract [6], nausea, profuse sweating, mental confusion, cyanosis and necrosis of human tissues by digestion [4,5]. Membrane separation process is an effective way for the extraction and recovery of methylene blue from wastewater produced by the textile industry (low energy consumption and use of simple efficiency techniques) [7].
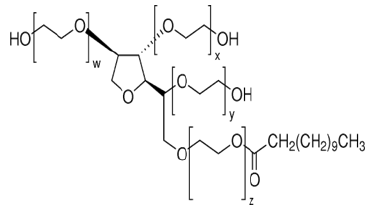
For this work, is to adopt the PIM membrane type for the extraction and recovery of methylene blue. The adopted polymer membrane was developed based on a mixed polymer support, polyvinylidene fluoride (PVDF), polyvinylpyrrolidone (PVP), and Tween 20 (TW20) as extractive agent (EA) (Figure 1).
Figure 1:Structure of extractive agent Tween20 (x+y+z= 20).
The aims are to study the influence of the initial concentration of methylene blue on the microscopic parameters (permeability P, flux J) and the macroscopic parameters (diffusion coefficient D* and association constant Kass). Secondly, we will study the influence of temperature on the determination of the activation parameters (Energy Ea, entropy ΔS#, enthalpy ΔH#) and the effect of activated carbon.
Material and Methods
Calculation
Permeability P and initial flux J0 are calculated from the following relationships [8,9]:
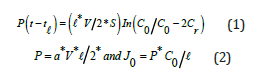
a: the slope experimental value of the straight lines -Ln(C0-2Cr) = f(t). ℓ: the membrane thickness. S: the membrane active area in contact with the aqueous solutions. V: the receiving phase volume. The microscopic parameters Kass and D* are determinate according to the following relationships:
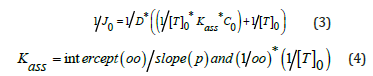
With p and OO are slope and intercept experimental values of the straight line
1/J0 = f (1/C0),[T]0 : the fixed total concentration of the extractive agent in the membrane phase.
The values of P and J0 are used to quantify the performance of PIM membrane. The initial flux (J0) is a function of extractive agent concentration in the organic phase of the membrane and evolves according to a saturation law with the initial concentration (C0) of the substrate. The values of Kass and D* are used to analyze the mechanism of substrate migration through the membrane phase based on the interaction of the substrate (S) with the extracting agent (T), and this migration step is the rate-determining step.
The activation parameters Ea, ΔH#, and ΔS# are related to the transition state of the interaction step of the substrate (S) with the extracting agent (T). Previous investigations [10,11] have demonstrated that the initial flux J0 evolves with temperature according to the Arrhenius relationship (Eq. 5).
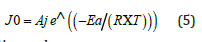
After linearization we have:
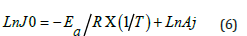
The slope (p) and intercept (oo) of the linear function Ln(J0)) = f(1/T) are used to calculate the values of Ea and Aj, which are used to determine the activation enthalpy (ΔH#) and entropy (ΔS#) of the transition state, according to the transition state theory (Eyring theory) (Eq. 7).
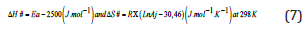
Therefore, these activation parameters (Ea, ΔH#, ΔS#) are important to understand the mechanism of these oriented processes through affinity polymer membranes. The activation parameters quantify the performance of adopted PIM and determine the nature of interactions between the substrate and the extracting agent.
Chemicals
All chemicals, reagents and solvents were pure commercial products (Aldrich, Fluka) of analytical quality. In addition, polyvinylidene fluoride (PVDF) obtained from Alfa Aesar was used as polymeric support.
Extraction experiments
The kinetic study of the studied process was performed using a device equipped with two glass compartments (“feeding” and “receiving”) [9,12,13]. The feeding phase contains methylene blue and the receiving phase contains distilled water. The membrane used was clamped between these two compartments. The device was immersed in a thermostatic bath with magnetic stirring in both compartments. Every hour, a negligible amount of water was taken with a micropipette to follow the evolution of the absorbance in the receiving phase as a function of time by ultraviolet-visible spectroscopy.
Results and Discussion
Characterization of the developed membranes by FTIR
The PVDF-PVP-Tw20 membrane was analyzed by Fourier transform infrared spectroscopy (FTIR) after being dried for 48 hours to remove residual water and solvent. A JASCO FT/IR-4600 spectrometer was used to record the FTIR spectra (Figure 2).
Figure 2:Fourier transform infrared-attenuated total reflection spectra of the membranes, PVDF support and PVDF-TW20.

Broad and clear absorption bands at 3450cm1 and 1700cm1 appear due to the alcohol (OH) moiety and the C=O vibration of Tw20, proving that the extractive agent was well inserted into the PVDF membrane surface.
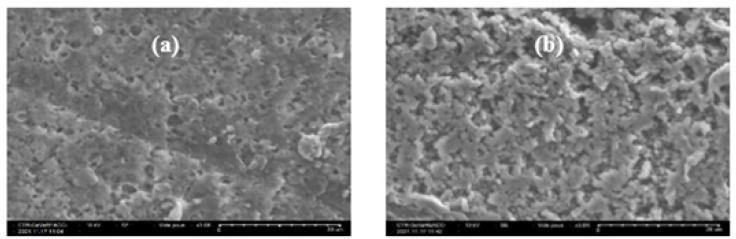
Characterization of the elaborated membranes by SEM
Figures 3 show the SEM surface of the support polymer PVDF alone and developed PVDF- TW20 membrane. These images very clearly indicate the different porous surfaces. The insertion of the extractive agent amphiphilic molecule changes the morphology of the membrane. The pore size increases as well as the porosity of the membrane.
Figure 3:(a) SEM image PVDF alone surface. (b) SEM image PVDF-TW20 surface.
Effect of initial substrate concentration C0 without and with activated carbon
In this section, the influence of the initial concentration of substrate C0 (0.001M, 0.0005M, 0.00025M, 0.00012M) on the evolution of the different parameters related to the facilitated extraction processes of BM through the PIM membrane (PVDFTW20) containing TW20 as extractant, under similar operating conditions (pH=3, T=298 K, support: PVDF) and then the influence of activated carbon on the same parameters and condition was examined by adding 0.2g of activated carbon in the receiver phase. Figure 4 show the evolution of the kinetic function - Ln (C0-2Cr) = f(t) [14].
According to the graphs shown in Figure 4, we clearly observe the linear straight lines of the variation of -Ln(C0-2Cr) as a function of time. From the slopes of these straight lines, the values of the parameters P and J0 are calculated and presented in Table 1.
Figure 4:Evolution of the kinetic function –Ln (C0-2Cr) = f(t) for the facilitated extraction BM without and with carbon. pH=3, and T=298 K.
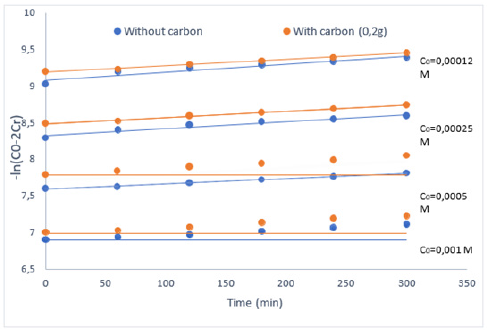
Table 1:Evolution of the membrane performance for the extraction-oriented processes of BM in the presence of the active carbon in the receiving phase, pH=3 and T=298K.

It can be seen that the permeability and initial flux values are related to the presence of activated carbon introduced in the receiving phase. Therefore, the activated carbon improves the performance of the membrane. This result is certainly due to the adsorption process of the BM molecules by the activated carbon in the receiving phase, which improves the performance of the elaborated membrane. To perform accurate interpretations and performance comparisons of these membranes, the Lineweaver- Burk method was adopted and the function 1/J0 = f(1/C0) have been studied (Figure 5), and in order to calculate and analyze the evolution of the microscopic parameters Kass and D* relating to the diffusion movement of the substrate molecules in the membrane phase. Using the values of the slopes and intercepts for the line segments obtained in Figure 5, the values of Kass and D* parameters could be determined, all obtained values are presented by the histograms in Figure 6.
Figure 5:Lineweaver-Burk representations curves (1/J0 = f (1/C0)) for the extraction-oriented processes of BM substrate without and with carbon through the PIM membrane pH=3 and T=298K.
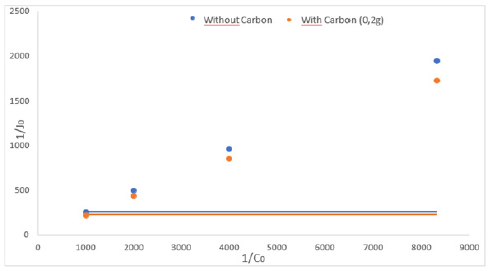
Figure 6:Evolution graphical representation of the microscopic parameters Kass and D* relating to facilitated extraction of BM.
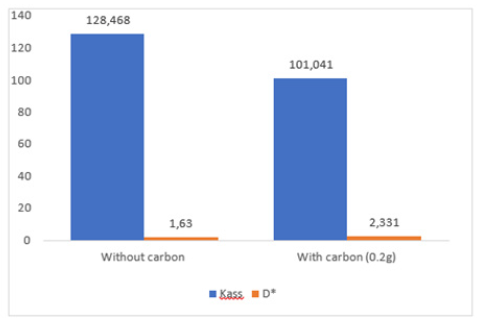
According to Figure 6, the activated carbon had a strong influence on the evolution of Kass and D*. It caused an increase in the diffusion coefficient and a decrease in the association constant, which shows that the association-dissociation reactions between substrate and extractive agent occur very quickly in the presence of activated carbon, causing a strong diffusion of the substrate molecules through the membrane.
Determination of activation parameters
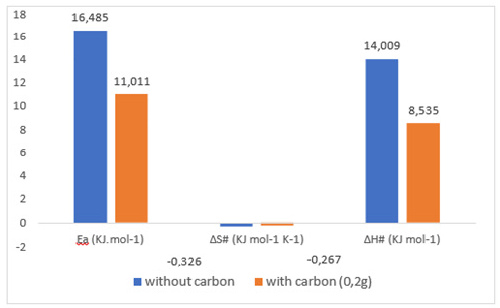
In order to examine the effects of the temperature factor on the performance of the membrane for the extraction of the BM substrate, studies were performed at three different temperatures (298, 303, and 308K) and at pH=3 (the optimal pH where the membrane is efficient). According to the calculation method described previously at T=298 K, the slopes of the line segments obtained by the representative of the kinetic function -Ln (C0-2CR) = f (t)) and the Lineweaver-Burk presentation 1/J0 = f (1/C0) allowed us to determine the values of the energetic parameters Ea, ΔH# ass,and ΔS#. These parameters were related to the transition state of the association/dissociation reaction, which is the kinetically determining step in the mechanism of facilitated extraction processes. The obtained results are presented by the opposite Histograms (Figure 7). From the acquired results, it can be seen that the passage of the transition state linked to the association - dissociation reactions require little energy (11,01 Kj/mol), especially in case of presence of activated carbon in the receiving phase. The more negative values of the ΔS# parameters mean that the movement of BM molecules across the membrane becomes increasingly organized and the interaction site (Substrate -TW20) changes from tridentate to bidentate in the presence of activated carbon
Figure 7:Energetic parameters without and with activated carbon.
Conclusion
According to the kinetic and thermodynamic results, the transport of MB substrate through the membrane phase takes place according to the formation of an intermediate entity of substrate with extractive agent. The developed membrane revealed a efficiency performance for the studied processes. The analysis of the results relating to the influence of activated carbon on the performance of the studied processes, showed a clear improvement in the processes of extraction and recovery of BM through the elaborated polymer membrane.
Funding
This work was supported by the Ministry of Higher Education and Scientific Research (MESRSFC) and the National Center of Scientific and Technical Research (CNRST). PPR2 project.
References
- (2008) Science and technology.
- Oz M, Lorke DE, Hasan M, Petroianu GA (2011) Cellular and molecular actions of methylene blue in the nervous system. Medicinal Research Reviews 31(1): 93-117.
- Gobi K, Mashitah MD, Vadivelu VM (2011) Adsorptive removal of Methylene Blue using novel adsorbent from palm oil mill effluent waste activated sludge: Equilibrium, thermodynamics and kinetic studies. Chemical Engineering Journal 171(3): 1246-1252.
- Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: A review. Journal of Hazardous Materials 177(1-3): 70-80.
- Uddin MT, Islam MA, Mahmud S, Rukanuzzaman M (2009) Adsorptive removal of methylene blue by tea waste. Journal of Hazardous Materials 164(1): 53-60.
- Low LW, Teng TT, Rafatullah M, Morad N, Azahari B (2013) Adsorption studies of methylene blue and malachite green from aqueous solutions by pretreated lignocellulosic materials. Separation Science and Technology 48(11): 1688-1698.
- Mourtah I, Touarssi I, Chaouqi Y, Sefiani N, Lebrun L, et al. (2019) Membrane oriented processes for elimination and recovery of Cr(VI) and Cr(III) through a grafted polymer membrane. Materials Today: Proceedings 13(3): 1039-1048.
- Hlaïbi M, Tbeur N, Benjjar A, Kamal O, Lebrun L (2011) Carbohydrate-resorcinarene complexes involved in the facilitated transport of alditols across a supported liquid membrane. Journal of Membrane Science 377(1-2): 231-240.
- Atmani EH, Benelyamani A, Mouadili H, Tarhouchi S, Majid S, et al. (2018) The oriented processes for extraction and recovery of paracetamol compound across different affinity polymer membranes. Parameters and mechanisms. European Journal of Pharmaceutics and Biopharmaceutics 126: 201-210.
- Benjjar A, Hor M, Riri M, Eljaddi T, Kamal O, et al. (2012) A new supported liquid membrane (SLM) with methyl cholate for facilitated transport of dichromate ions from mineral acids: parameters and mechanism relating to the transport **. J Mater Environ Sci 3(5): 826-839.
- Bacharach AL, Robinson FA (2022) The theory of rate processes, UTC.
- Mouadili H, Majid S, Kamal O, ElAtmani ELH, Touaj K, et al. (2018) New grafted polymer membrane for extraction, separation and recovery processes of sucrose, glucose and fructose from the sugar industry discharges. Separation and Purification Technology 200: 230-241.
- Hor M, Riad A, Benjjar A, Lebrun L, Hlaïbi M (2010) Technique of supported liquid membranes (SLMs) for the facilitated transport of vanadium ions (VO2+). Parameters and mechanism of the transport process. Desalination 255(1-3): 188-195.
- Mechnou I, Mourtah I, Raji Y, Chérif A, Lebrun L, et al. (2021) Effective treatment and the valorization of solid and liquid toxic discharges from olive oil industries, for sustainable and clean production of bio-coal. Journal of Cleaner Production 288: 125649.
© 2022 M Hlaïbi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






