- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Biosynthesis of the Fe3O4 Nanoparticles Using Acacia Nilotica Leaf Extract and their Effect on Degradation of Congo Red Dye in Aqueous Solution
Cheera Prasad K, Sreenivasulu V, Govinda S, Himageerish kumar K, Deepa T, Vasantha Jyothi NVV* and Venkateswarlu P*
Department of Chemistry, Sri Venkateswara University, India
*Corresponding author: Venkateswarlu P and Vasantha Jyothi NVV, Biopolymers and Thermo Physical Laboratories, Department of Chemistry, Sri Venkateswara University, Tirupati 517 502, Andhra Pradesh, India
Submission: February 02, 2018; Published: February 16, 2018

ISSN: 2578-0271 Volume1 Issue3
Abstract
Iron oxide magnetic nanoparticles (Fe3O4 MNPs) were synthesized using Acacia nilotica leaf extracts as reducing and capping agent. Their morphology, structure and size were confirmed by UV-visible (UV-vis), Fourier Transform Infrared spectroscopy (FTIR), X-ray diffraction (XRD), transmission electron microscope (TEM) and vibrating magnetometer sample (VSM). The synthesized Fe3O4 MNPs results in mostly spherical particles with diameters ranging from 16 to 20nm. The catalytic properties of Fe3O4 MNPs for degradation of Congo red (CR) dye in aqueous solution have been studied by UV-vis spectroscopy. The results show that the synthesized high surface area Fe3O4 MNPs has an excellent adsorption performance.
Keywords: Fe3O4 MNPs; XRD; VSM
Introduction
It is well known that organic dyes are used in a broad range of industries, especially in textiles. Most of synthetic textile dyes are mutagenic and/or carcinogenic and belong to the most dangerous pollutants [1-3]. Although conventional chemical and physical techniques such as precipitation, adsorption, and ozonation have been employed for the decolorization of dye effluents, they possess inherent limitations such as high cost, formation of hazardous by-products, and intensive energy requirements [4-6]. It is widely acknowledge that there is a growing need for more environmentally acceptable processes in the catalytic reduction of colorless organic pollutants.
Various synthesis techniques including sol-gel, quick precipitation, sonochemical, electrochemical, solid state reaction, microwave irradiation and alcohothermal synthesis have been applied to the preparation of metal nanoparticles [7-15]. However, most of these methods suffer from some disadvantages such as harsh reaction conditions, high temperature and long reaction times, the use of expensive, hazardous and toxic capping agents or stabilizers to protect the size and composition of the NPs, the environmental pollution caused by utilization of organic solvents and low yields of the products.
Therefore, the green synthesis has been coming up with as a cost effective and environmentally friendly and alternative to chemical and physical methods. The previous reports suggest that some of the reports are in availability on green synthesis of iron oxide (Fe3O4) nanoparticles [16-20]. However, in this study, we report acacia nilotica leaves extract as capping and reducing agent for synthesis of Fe3O4 MNPs. In the next step, based on our interest in metal NPs catalyzed reactions, herein we now report a simple and inexpensive protocol for the reduction of CR in aqueous medium at room temperature with sodium borohydride as hydrogen source and in the presence of the Fe3O4 NPs as separable and reusable catalyst. The catalyst can easily be recovered using an external magnet and reused several times without significant loss of its catalytic activity.
Materials and Methods
Preparation of acacia nilotica leaf extract
10g of dried leaf powder of acacia nilotica was added to 250mL double distilled water then the mixture was stirred at 80 °C for 1 hr to obtain the extract. The prepared extract was centrifuged at the speed of 8000rpm, and filtrated then kept at refrigerator to for further use.
Synthesis of iron oxide nanoparticles
0.1M of Ferrous sulphate was prepared using 100mL of deionized water. To this precursor solution and 10mL of plant extract were mixed. 10mL of NaOH (0.1M) was added drop wise to mixture of solution (precursor solution and plant extract) under continuous stirring. The mixture was stirred at 60 °C for 2 h. After that, the supernatant was discarded and the pellet was dried in hot air oven. A brownish black color powder was obtained and it was kept in the sterile bottles for the further investigation.
Activity of the Fe3O4 NPs for the reduction of CR in water
To further confirm the catalytic performance of the Fe3O4 NPs, the catalyst was applied to the reduction of CR with 25mL of fresh NaBH4 aqueous solution (5.3*10-3M). The reduction reaction can be easily monitored by UV-vis absorption spectroscopy. As shown in figures, when Fe3O4 NPs was added into the solution containing 1.44x10-5M of CR and 5.3*10-3M of NaBH4, the intensity of the strong absorption peak at 493nm gradually decreased and within 30 min the whole peak disappeared.
Characterization
The as synthesized Fe3O4 MNPs was characterized by X-ray diffract meter Seifert 3003 TT with CuKα radiation having a wave length of 1.52 Å. Surface morphological and size distribution was conducted with transmission electron microscope (TEM). High- resolution TEM (HRTEM) images were obtained on JEOL JEM- 2100 machine with an accelerating voltage of 200kV. The magnetic measurements were recorded at room temperature using vibrating sample magnetometer (VSM, LKSM-7410). Fourier transform infrared (FTIR) spectra were recorded using with thermo Nicolet FTIR-200 thermo electron corporation spectrophotometer by KBr pellet method at room temperature.
Results and Discussion
FT-IR analysis
Figure 1: FT-IR spectrum of Fe3O4 MNPs samples.
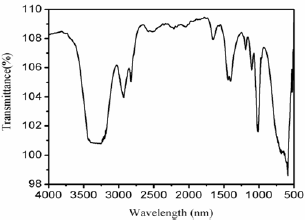
Figure 1 shows the FT-IR spectra of Fe3O4 MNPs. FT-IR spectra of Fe3O4 MNPs show peaks at 3330, 2956, 1660, 1450 and 1126cm-1. The sharp and intense band at 3330 is ascribed to the O-H stretching vibration corresponding to the polyphenol groups. The band at 2956cm-1 can be assigned to methyl C-H stretching vibrations and the one at 1660cm-1 is due to the C=O stretching of aldehydes in the leave extract. The other peaks observed are due to stretching C=C aromatic ring and C-OH stretching vibrations. In addition to other bands a sharp peak ~579cm-1 appears in Fe3O4 composite sue to the characteristic Fe-O stretching vibration [21,22].
Powder XRD analysis
Figure 2: The powder XRD pattern of the Fe3O4 MNPs sample.
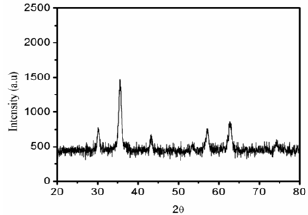
Figure 2 shows the powder XRD patterns of Fe3O4 MNPs. The prepared Fe3O4 MNPs were highly crystalline with diffraction peaks corresponding to the face centered cubic (fcc) phase of metallic iron. The XRD sample recorded for Fe3O4 MNPs revealed five intense peaks in the whole spectrum of 20 values ranging from20 to 80°. A number of diffraction peaks were identified at 20 values of 29.7, 35.02, 42.5, 56.2 and 61.7°, which are well corresponding to the (220), (311), (400), (511) and (440) planes face-centered cubic (fcc) phase of metallic iron. The particle size of the Fe3O4 MNPs were calculated using Debye-Scherrer equations given formula D=0.89A/pcos0 where D is the average particle size, A is the wave length of the Cu-Ka irradiation, p is the full width at half maximum intensity of the diffraction peak and 0 is the diffraction angle for the respective peaks of the Fe3O4 MNPs which was around ~26nm were good in agreement with TEM results.
Morphology and particle size distribution analysis
Figure 3: TEM images of the Fe3O4 nanoparticles.
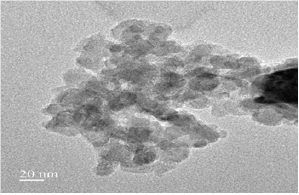
TEM analysis was used to visualize the size and shape of Fe3O4 MNPs formed and the images show that they are relatively uniform in diameter and spherical in shape (Figure 3). However, the TEM suggested that to measure particles have a single size distribution with a characteristic diameter of about 16-20nm and diagonal lengths of the individual particles are considered.
Figure 4: M-H hysteresis loop of the Fe3O4 MNPs sample measures at 300 K.
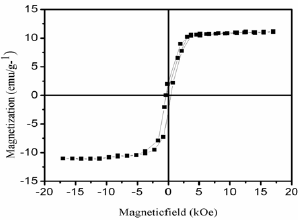
The magnetic behavior of the materials is strongly dependent on shape, morphology and size which are greatly influenced by the synthetic procedure. Figure 4 depicts magnetic hysteresis loop (magnetic field, H versus magnetic moment, M) of Fe3O4 nanoparticles at 300K. The M-H clearly shows ferromagnetic behavior of the sample. From Figure 4 we obtain magnetic parameters viz. saturation magnetization (Ms), remant magnetization (Mr) and coercivity (Hc) values are 11.1emu/g, 1.92emu/g and 342 Oe, respectively. These values are we supported with the earlier reported Fe3O4 nanoparticles [23-24].
Adsorption study
Figure 5: UV-vis spectrophotometer spectrum of congo red dye in the presence of NaBH4 and Fe3O4 MNPs with different time intervals 0, 10 and 30min.
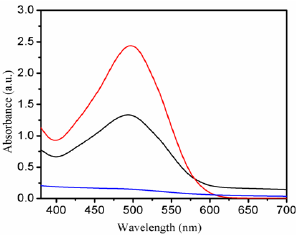
Further, we explored the reduction of CR dye in the presence of NaBH4 at room temperature by using the Fe3O4 MNPs via electron transfer of BH4 - ions [25-28]. The reaction was studied by recording the time dependent UV-vis absorption spectra of the mixture using a spectrophotometer. In Figure 5 the absorption peaks at 493nm corresponding to CR dye, after the addition of aqueous solution of NaBH4 and Fe3O4 MNPs as catalyst the whole absorbance peak almost disappeared after 30 min, depending upon the dye. The reducing reaction was not observed in the absence of catalyst, even in the existence of excess amounts of NaBH4. Biosynthesized nanocatalyst exhibited supreme catalytic activity through reduction of organic dyes within 30 min for CR. The results show that the Fe3O4 MNPs is a more efficient catalyst with respect to reaction time and conversion than previously reported ones.
Conclusion
In summary, we have demonstrated for the first time a novel green synthesis of iron oxide nanoparticles using acacia nilotica leaf extract. The iron oxide crystal structure and phase has explored using powder XRD and the morphology was determined by TEM analysis. The synthesized Fe3O4 MNPs shows fcc structure and particle size of ~26nm. Furthermore, Fe3O4 MNPs exhibit ferromagnetism with saturation magnetization value of ~11.1emu/g. Finally, the prepared Fe3O4 MNPs showed an excellent ability to remove organic pollution from wastewater.
Acknowledgement
The authors thank to IIT, Madras for providing the instrumentation facility and one of the author Ch. Prasad is grateful acknowledge to CSIR-UGC for financial assistance under JRF and SRF scheme.
References
- Banat IM, Nigam P, Singh D, Marchant R (1996) Bioresource Technology 58: 217.
- Martinez-Huitle CA, Brillas E (2009) Applied Catalyst B 87: 105.
- Vidhu VK, Philip D (2014) Micron 56: 54.
- Manu B, Chaudhari S (2002) Bioresource Technol 82: 225.
- Patel R, Suresh S, Hazard J (2006) Materials B 137: 1729.
- Devi LG, Kumar SG, Reddy KM, Munikrishnappa C, Hazard J (2009) Mater 164: 459.
- Guang C, Yong JT, Wei L, Jiang SL, Jun L, et al. (2005) Metallic Function. Materials 12(3): 18.
- Qing CC (2005) Fine Chem 22(6): 417.
- Xin LG, Zheng TS (2005) Applied Chemical Industry 34(10): 615.
- Xiao LW, Bin SX, He LY, Yi X (2005) China Surface Eng 18(5): 24.
- Feng H, Zheng YZ, Yao FX, Wu XW, Ya FH, et al. (2000) Acta Metallurgica Sinica 36(6): 659.
- Qi FW, Qi XZ (2003) Non-ferrous Smelting. 6(3): 10.
- Li JH, Cai XH, Yong HW (2005) Journal of Gansu Lianhe University (Natural Science) 19(4): 49.
- Ling HT, Feng SL (2005) Journal of Nanjing Institute Technol0gy (Natural Science Edition) 3(1): 6.
- Suleiman M, Mousa M, Hussein A, Hammouti B, Hadda TB, et al. (2013) Journal of Materials and Environmental science 4: 792.
- Cai Y, Shen Y, Xie A, Li S, Wang X, et al. (2010) Magnetic Materials 322: 2938.
- Lu W, Shen Y, Xie A, Zhang W, Magn J (2010) Magnetic Materials 322: 1828.
- Venkateswarlu S, Rao YS, Balaji T, Prathima B, Jyothi NVV (2013) Materials Letters 100: 241.
- Hoag G, Collings J, Holcomb J, Hoag J, Nadagoud M, Varma R (2009) Journal of Materials Chemisrty 19: 8671.
- Chrysochoou M, Johnston CP, Dahal G, Hazard J (2012) Materials 201: 33.
- Prasad CH, Karlapudi S, Sellola G, Venkateswarlu P (2016) Journal of Molecular Liquids 221: 993.
- Prasad CH, Karlapudi S, Sellola G, Venkateswarlu P (2017) Journal of Alloys and Compouds 700: 252.
- Prasad CH, Yuvaraja G, Venkateswarlu P (2017) Journal of Magnetism and Magnetic Materials 424: 376.
- Prasad CH, Karlapudi S, Venkateswarlu P, Bahadur I, Kumar S (2017) Journal of Molecular Liquids 240: 322.
- Ghosh BK, Hazra S, Nak B, Ghosh NN (2015) Powder Technol 269: 371.
- Zhang P, Sui Y, Wang C, Wang Y, Cui G, et al. (2014) Nanoscale 6: 5343.
- Rostami VA, Nasrollahzadeh M, Alizadeh M (2016) Journal of Colloid Interface Science 470: 268.
- Atarod M, Nasrollahzadeh M, Sajadi SM (2016) Journal of Colloid Interface Science 462: 272.
© 2018 Cheera Prasad K, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






