- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Diffuse Midline Glioma, H3 K27M-Mutant of the Pons - Multidisciplinary Appro
Reinas Rui1*, Carvalho Bruno2, Nunes Susana3, Sampaio Luísa4, Silva Roberto5, Lima Jorge6, Osório Lígia7, Gilda Costa Maria João3, Vaz Rui8 and Pereira Josué9*
1Department of Neurosurgery, Portugal
2Consultant of Neurosurgery, Portugal
3Consultant of Pediatric Oncology, Portugal
4Assistant of Neuroradiology, Portugal
5Consultant of Pathology, Portugal
6Biologist, Faculty of Medicine, Portugal
7Consultant of Radiation Oncology, Portugal
8Head of Department of Neurosurgery, Portugal
9Consultant of Pediatric Neurosurgery, Portugal
*Corresponding author: Rui Reinas, Department of Neurosurgery, Portugal
Submission: November 03, 2021;Published: November 16, 2021

ISSN 2637-7748
Volume4 Issue4
Abstract
Background: Diffuse Midline Glioma (DMG) H3 K27M-mutant of the pons is the most common brainstem tumor of childhood, leading to poor outcome. Radiotherapy is the only proved and consensual therapeutic option that just offers temporary benefit, while co-adjuvant chemotherapy is still controversial. Although MRI allows for the diagnosis of DMG, biopsy is the gold standard as it offers additional molecular biology information that can be useful for future targeted immune or chemotherapy, improving diagnostic accuracy, prognostic stratification and potentially unveiling more treatment options.
Case: A 6-year-old girl presented with a 6-month history of vomits, ataxia with frequent falls, left peripheral facial palsy and headache along with irritability. Brain MRI showed an intra-axial lesion located in the mesencephalon and pons, hypointense in T1, hyperintense in T2 and FLAIR, without contrast enhancement. The dysmorphic pons partly surrounded the basilar artery, obstructing the upper part of the 4th ventricle and aqueduct, with supratentorial ventricular dilatation. A trans-frontal stereotactic biopsy of the lesion was performed followed by an Endoscopic Third Ventriculostomy (ETV), through two different burr holes and transcortical approaches. Histology and biology confirmed the diagnosis of H3 K27M-mutant DMG.
Conclusion: Biopsy is an invasive procedure; however, it should be offered to children with presumptive imaging of DMG. It shows low morbidity, confirms the diagnosis and has potential utility for research on targeted chemotherapy or immunotherapy for secondary treatment approach.
Keywords: Diffuse Midline Glioma; Stereotactic Biopsy; Molecular Diagnosis; Targeted Chemotherapy
Abbreviations: BBB: Blood-brain Barrier; CSF: Cerebrospinal Fluid; CT: Computerized Tomography; DMG: Diffuse Midline Glioma; DNA: Deoxyribonucleic Acid; ETV: Endoscopic Third Ventriculostomy; MRI: Magnetic Resonance Imaging; PFS: Progression-free Survival; RNA: Ribonucleic Acid; VP: Ventriculo Peritoneal; WHO: World Health Organization
Introduction
Diffuse Midline Glioma (DMG) H3 K27M-mutant of the pons is a rare malignant brain tumor of childhood. However, it is the most common subtype (80-85%) of the of diffuse midline gliomas [1,2]. The mean age at diagnosis ranges from 5 to 11 years-old, with no clear gender predominance. Despite decades of research, the outcome remains dismal: the overall survival is 9-12 months, with less than 10% of patients alive at 2-years follow-up [3-5]. Most patients present with signs and symptoms of brainstem dysfunction [6] with 22-89% developing obstructive hydrocephalus [7]. The classic triad includes ataxia, long tract signs and multiple cranial nerves deficits. A rapid progression is usually observed, with symptoms evolving over 1-2 months [5]. Radiologically, DMG presents on MRI as a lesion which typically occupies two thirds of the pons, showing hypointensity in T1 and hyperintensity in T2. A grim survival correlates with contrast enhancing [8,9]. The encasement of the basilar artery is a classic characteristic present in DMG, and it may also protrude into the aqueduct/fourth ventricle, causing ventricular dilatation. For decades, the topography of DMG, coupled with typical MRI characteristics, based the diagnostic criteria enough to perform therapy. However, this diagnostic approach led to an insufficient insight into the growing molecular pathogenesis knowledge of DMG. The paradigm started changing in the late 1990’s with Puget et al. [10], that questioned the lack of safety of biopsies in the brainstem needed to obtain useful clinical biomarkers. Further evidence points towards safety and cost-effectiveness of biopsies in this topography [11,12]. At this moment, radiotherapy remains the cornerstone of DMG treatment [13,14], with little progress towards effective chemotherapy [4]. We report on the clinical case of a patient that underwent a biopsy of DMG, with focus on surgical technique and risks, results of histologic and molecular, ongoing therapies and review of current evidence on the topic.
Case Presentation
A 6-year-old girl presented with 6 months of headache, vomits, ataxia and left peripheral facial palsy along with irritability in the days prior to admission. Her mother reported an increasing number of falls in that period without objective motor deficit, with a CT scan performed three months before described as without evidence of any obvious intracranial lesion. Brain MRI showed a huge intra-axial lesion in the pons and mesencephalon, hypointense in T1, hyperintense in T2 and FLAIR (Figure 1), without contrast enhancement, partly encasing the basilar artery without stenosis, and compressing the aqueduct/4th ventricle with slight ventricular dilatation. Cerebellar tonsils were located at foramen magnum level. Whole-spine MRI did not disclose any other lesion. Considering a probable diagnosis of DMG, it was decided to undertake a transfrontal stereotactic brainstem biopsy, using a Leksell® frame (Elekta AB®, Stockholm, Sweden). The biopsy planning was made four days before the procedure through a volumetric FLAIR and T1- Gadolinium imaging merged with the stereotactic CT protocol on a neuro-navigation workstation (Stealth Station S8, Medtronic®, Dublin, Ireland) (Figure 2). The target area was located on the right upper-half of the mesencephalon, midway in the antero-posterior axis, on a small area with diffusion restriction in order to avoid under sampling. A right-sided frontal burr hole was done to allow the stereotactic biopsy three small tumor fragments were obtained with a Sedan biopsy needle (and sent for histological study) without apparent bleeding. The next step was the endoscopic third ventriculostomy (ETV) (Minop® system, optics 0 degrees, Aesculap Inc; Tuttlingen, Germany) that also allowed the collection of 10 cc of CSF for standard study (cytology, biochemistry work and microbiology), tumoral germinative markers (β-HCG and α-fetoprotein) and molecular studies.
Figure 1: (A) Intra-axial pontomesencephalic lesion, hypo/isointense in T1, (B,C) hyperintense in T2 and FLAIR,
(D,E) with foci of restriction to diffusion. The basilar artery is almost completely encased by the tumor, as it ascends
in the interpeduncular fossa. There is marked compression of both the aqueduct and 4th ventricle, with subsequent
supratentorial ventricular dilation.
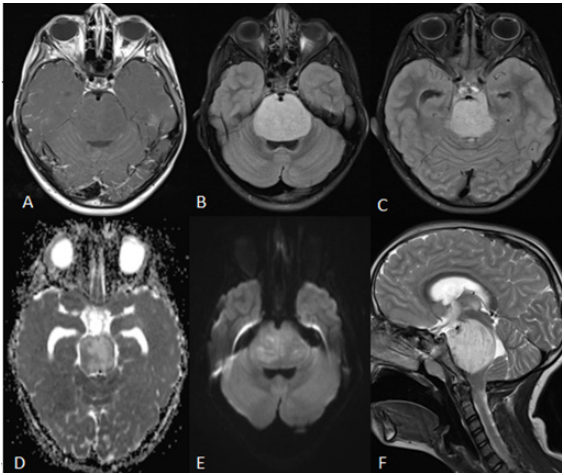
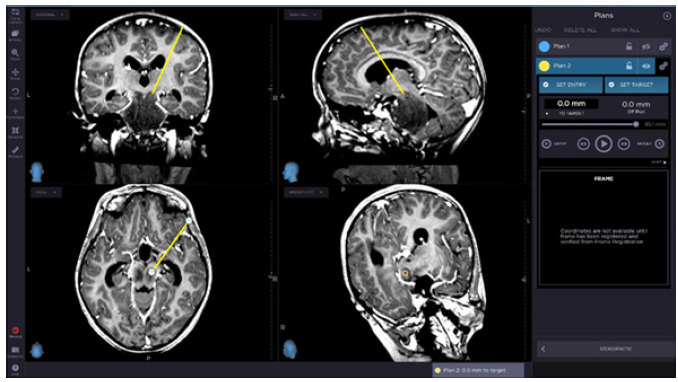
Figure 2: Surgical planning for the biopsy target set for lesion on the right side of the mesencephalon, attempting to
avoid lemnisci, periaqueductal nuclei, and medial longitudinal fascicle. A small area with diffusion restriction was
chosen as target to avoid under sampling.
The procedure was uneventful, with the child waking up without new neurological deficits. Control CT scan did not reveal adverse events, namely hematomas the child was transferred from the Pediatric ICU to the Pediatric Oncology ward on day one postoperative. She displayed less irritability when compared to preventriculostomy status. Histology and biology exam confirmed Diffuse Midline Glioma, H3 K27M-mutant (Figure 3). The molecular study was performed using the NGS panel Oncomine TM Childhood Cancer Research Assay to search for molecular alterations at the DNA and RNA level. The study showed the presence of the following mutations: p. (Lys27Met) in HIST1H3B; p. (Cys135Gly) in TP53; and p. (Gly328Trp) in ACVR1. No gene rearrangements or CNVs were found. After ventriculostomy and biopsy, steroids were discontinued and the patient started complementary therapy after multidisciplinary discussion - a 12-week induction cycle of Nimotuzumab 150mg/m2 and Vinorelbine 20mg/m2 once per week, followed by a maintenance scheme with both drugs (Nimotuzumab 150mg/m2 and Vinorelbine at 25mg/m2), once every 2 weeks, up to 52 weeks or progression. Three weeks into induction, the child started local radiotherapy [15,16] 54Gy in 30 fractions of 1.8Gy/day over 6 weeks with 3DCRT technique. She tolerated the treatment well in an outpatient basis (despite a brief pause for an asymptomatic SARS-COV2 infection), with both clinical and image improvement, namely without ataxia or left peripheral facial palsy, reduction of the tumor size and edema and no hydrocephalus nor need for VP shunt at seven months of followup (Figure 4).
Figure 3:3A. Diffuse glioma with monomorphic tumour cells (400X–HE;). 3B. Strong nuclear staining for K27Mmutant
H3 is present in tumoral cells (400X).
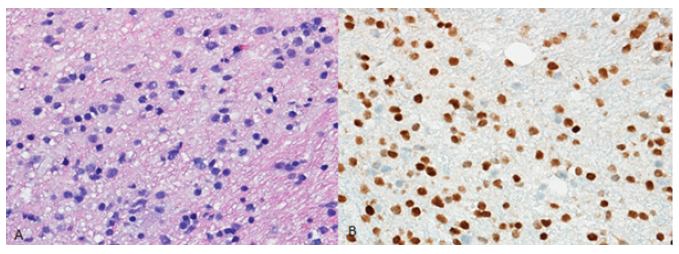
Figure 4: At 4 months after biopsy, and after complementary therapy, the tumor was reduced in size, with no foci
of enhancement after gadolinium, less edema and no hydrocephalus. Basal cisterns are less compressed, and the
basilar artery is less engulfed by the tumor. Significantly, there is minimal local impact from the biopsy on the right
side of the mesencephalon.

Discussion
DMG is uniformly associated with a poor outcome (survival rates are consistently very low, from 9 to 12 months), which may be partially attributed to decades of delay in molecular biology knowledge the management strategy was for a long time based on MRI findings in detriment of biopsy tissue diagnosis [17]. In the past, the main argument against biopsy was the high risk of complications stemming high density of tracts and nuclei located in the pons and mesencephalon. It was also argued that regarding functional and survival outcome it was not cost-effective to risk morbidity from this invasive procedure in patients whose MRI features were rather typical [18,19]. However, according to the literature, stereotactic biopsy of brainstem lesions is nowadays feasible and harbors low morbidity. Kickingereder et al. [11] reported 7.8% of morbidity in their meta-analysis of 1480 stereotactic biopsies of brainstem tumors with only 1.7% of permanent deficits. This study included both trans frontal and trans cerebellar approaches, and the authors found that greater experience led to lower incidence of complications. Gupta et al. [12] in their cohort of 50 patients with MRI diagnosis of DMG who underwent trans cerebellar stereotactic biopsy presented 5 adverse events with only one leading to a persistent deficit (dysarthria). The morbidity for stereotactic brainstem biopsy tends to be low, however it is still debatable which route is the best. Dellaretti et al. [18] reported slightly higher success rates with trans frontal versus trans cerebellar approaches (95.1 vs 84.2%), but with similar low morbidity [19]. Chen et al. [19] reported low morbidity for both approaches in their small cohort [20] and Hamisch et al. [20] described 0.6% morbidity and 0.6% mortality in a meta-analysis which included 735 biopsies, showing no correlation between outcome measures and the type of approach. Usually, a trans frontal approach is chosen for lesions centered in the mesencephalon, while trans cerebellar tends to be preferred for pontine lesions (occasionally reaching the medulla or the cerebellar peduncles) [21-24]. Gonçalves et al. [22] described that the angle of the tentorium made the mesencephalon more easily accessible through a trans frontal approach. This author also pointed that the trans cerebellar approach was the shortest route for the pons but was associated with a higher risk of complications [22]. Pincus et al. [23] also stated the same anatomical criteria when deciding the approach for brainstem biopsies.
Assuming the safety and feasibility of stereotactic biopsy for brainstem lesions, the next step is to secure the accuracy of tissue diagnosis. This can be improved when MRI images are fused with the pre-operative CT scan [25-27] allowing a greater precision in trajectory and tissue biopsy yield [28]. Some authors caution [29] against potential errors during registration and fusion of images, which can be sought and corrected in the workstation. MRI-CT fusion images improve navigation between eloquent structures and nuclei, avoiding eloquent areas, which is essential to prevent additional morbidity. The role of PET scan for biopsy planning is still under study. Goda et al. [29] reported efficacy in detecting high-grade tumors with 18F-FDG, but a sensitivity below 50% for low-grade brainstem tumors. Kossatz et al. [30] describes that 18F-PARPi may hold greater potential in aiding planning of a biopsy, but more studies are required. Lack of availability of the original CT scan made 3 months prior to admission prevents a retrospective re-assessment but its description as without lesions signal the low sensitivity of CT to DMG [31]. A high degree of suspicion is necessary in order to avoid a false negative result. Tissue biopsy allowing pathology and molecular studies could be a key factor in reversing the unfavorable prognosis of DMG. Histone H3 gene mutations are among the most common, affecting regulation of gene transcription and DNA methylation [32]. K27M mutation in H3F3A, HIST1H3B and HIST1H3C genes is used to classify these tumors according to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System [32], and it is present in over 80% of DMG patients [31]. Detection of the H3K27M mutation in CSF cytology is under study [33], underlining the importance of collecting CSF samples. The case here reported harbors the K27M hotspot mutation in HIST1H3B, which is usually observed in 25% of DMG patients [34]. This mutation was found together with the G328W in ACVR1, a hotspot gain-of-function mutation that is intimately associated with the HIST1H3B K27M in DMG. The tumor also showed a TP53 mutation, which is a common finding, particularly in H3F3A-mutant DMG [35]. These mutations may be potential targets for new drugs. Targeting oncogenic transcription with bromodomain inhibition, blocking CDK7 or using inhibitors of ALK2 (encoded by ACVR1 gene) all of which are options under study, to create therapies that may improve survival outcomes [33-37]. Thus, sharing histological and molecular results with multicentric DMG registries (International DIPG Registry (IDIPGR), or the European Society for Pediatric Oncology DIPG Registry (SIOPE-DIPGR) will help to overcome the rarity of this tumor and years of delay in research [38,39]. Targeted chemotherapy may be the key to improve DMG outcomes. Li et al. [39] found that 6 out of 11 DMG patients presented with overexpression of EGFRvIII, a surface molecule rarely present in normal tissue40. Other authors [40-42] reported even stronger associations, with up to 80-85% positivity. This has led to the inclusion of nimotuzumab, an anti- EGFR monoclonal antibody, in chemotherapy regimens used to treat DMG patients. Fleishhacker et al. [42] reported on the efficacy of adding nimotuzumab to radiotherapy, with 39.6% of Progression Free Survival (PFS) at 6 months and median Overall Survival (OS) of 9.4 months. Massimino et al. [16] also described improved outcomes with the addition of nimotuzumab to vinorelbine and radiotherapy (response in 96% of patients, PFS and OS of 30±10 % and 76±9 % at 1 year, respectively; at 2 years, OS was 27±9 %). Our multidisciplinary group used the same regimen described by Massimino et al. [16], with our patient displaying a favorable progression thus far. Its use in an outpatient setting is associated with very few side effects and great tolerability. EGFRvIII is but one example of the benefits of histological and molecular studies. In time, other targets for chemotherapy may be identified, further improving survival and quality of life for DMG patients. Convection- Enhanced Delivery (CED), currently under study, has the potential to overcome the Blood-Brain Barrier (BBB) by directly delivering homogenous drug concentrations to the site of disease, significantly reducing systemic toxicity [43-45]. A crucial procedure which may improve immediately the clinical status in DMG patients is the treatment of obstructive hydrocephalus, a critical secondary event [7]. Fonseca et al. [45] describes that up to 55% of DMG patients may present with ventriculomegaly. The majority are asymptomatic and do not require surgical treatment. However, in the group that becomes symptomatic, CSF diversion improves survival outcomes [46]. The role of ETV in this subgroup is well established, as is both safe and effective in the pediatric population, avoiding a shunt “disease”. Guida et al. [46] in their systematic review of 6 studies reported 86% (30/35 patients) of sustained clinical improvement with ETV in DMG patients, with no associated morbidity. Considering the poor prognosis, it is important to treat these patients early and assertively. The uneventful course of the case described above allowed an early start of adjuvant treatments, a key goal for choosing minimally invasive procedures. The possibility of shortening duration of corticosteroids therapy is an important additional advantage, enabling better efficacy of complementary treatment, both chemo and radiotherapy, with concomitant avoidance of the well-known negative effects of prolonged steroids use.
Conclusion
Stereotactic brainstem biopsy is safe and should be considered in suspected DMG on MRI. Planning for the stereotactic procedure with neuronavigational using MRI-CT fusion increases precision of tissue sampling and lowers the morbidity of the procedure, resulting in increased effectiveness. Histological and molecular diagnosis is of high importance to understand DMG pathogenesis and unravel more effective targeted therapies against DMG when progression occurs.
Study Funding
This work was partially financed by a grant from Lions Club Portugal and from Liga Portuguesa Contra o Cancro.
References
- Aziz BR, Monje M (2019) Diffuse intrinsic pontine glioma: molecular landscape and emerging therapeutic targets. Curr Opin Oncol 31(6): 522-530.
- Vanan MI, Eisenstat DD (2015) DMG in children - what can we learn from the past? Front Oncol 21(5): 237.
- Cocaa HA, Cebulaa H, Benmekhbia M, Chenardb MP, Entz WN, et al. (2016) Diffuse intrinsic pontine gliomas in children: Interest of robotic frameless assisted biopsy. A technical note. Neurochirurgie 62(6): 327-331.
- Gwak HS, Park HJ (2017) Developing chemotherapy for diffuse pontine intrinsic gliomas (DMG). Crit Rev Oncol Hematol 120: 111-119.
- Bredlau AL, Korones DN (2014) Diffuse intrinsic pontine gliomas: treatments and controversies. Adv Cancer Res 121: 235-259.
- Louis DN (2016) WHO pathology and genetics of tumours of the central nervous system. WHO press 2016 pp.66-68.
- Guida L, Frank ERFE, Massimino M (2018) Safety and efficacy of endoscopic third ventriculostomy in diffuse intrinsic pontine glioma related hydrocephalus: a systematic review. World Neurosurg 29(18): S1878-8750.
- Poussaint TY, Kocak M, Vajapeyam S (2011) MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro oncol 13(4): 417-427.
- Jansen MH, Veldhuijzenvan ZSE, Sanchez AE (2015) Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro oncol 17(1): 160-166.
- Puget S, Blauwblomme T, Grill J (2012) Is biopsy safe in children with newly diagnosed diffuse intrinsic pontine glioma? Am Soc Clin Oncol Educ Book pp. 629-633.
- Kickingereder P, Willeit P, Simon T, Ruge MI (2013) Diagnostic value and safety of stereotactic biopsy for brainstem tumors: a systematic review and meta-analysis of 1480 cases. Neurosurgery 72(6): 873-881.
- Gupta N, Goumnerova LC, Manley P (2018) Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol 20(11): 1547-1555.
- Dellaretti M, Câmara BBA, Ferreira PHPB, José Batista, Arantes RME (2020) Impact of histological diagnosis on the treatment of atypical brainstem lesions. Sci Rep 10(1): 11065.
- El Khouly FE (2019) Diagnostics and treatment of diffuse intrinsic pontine glioma: where do we stand? J Neurooncol 145(1): 177-184.
- Massimino M, Biassoni V, Miceli R (2014) Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol 118(2): 305-312.
- Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, et al. (1993) Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the children's cancer group current perspective. Neurosurgery 33(6): 1026-1029.
- Dellaretti M (2012) Diffuse brainstem glioma: Prognostic factors. J Neurosurg 117(5): 810-814.
- Dellaretti M, Reyns N, Touzet G, Dubois F, Gusmão S, et al. (2012) Stereotactic biopsy for brainstem tumors: comparison of transcerebellar with transfrontal approach. Stereotact Funct Neurosurg 90(2): 79-83.
- Chen SY, Chen CH, Sun MH, Lee HT, Shen CC (2011) Stereotactic biopsy for brainstem lesion: comparison of approaches and reports of 10 cases. Journal of the Chinese Medical Association 74: 110-114
- Hamisch C, Kickingereder P, Fischer M, Simon T, Ruge MI (2017) Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: a systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr 20(3): 261-268.
- Gonçalves FAJ, Herculano CM, Pimentel J (2003) Stereotactic biopsies of focal brainstem lesions. Surg Neurol 60(4): 311-20.
- Goncalves FA (1991) Stereotactic anatomy of the posterior cranial fossa; a study of the transcerebellar approach to the brainstem. Acta Neurochir (Wien) 113(3-4): 149-165.
- Pincus DW, Richter EO, Yachnis AT, Bennett J, Bhatti MT, et al. (2006) Brainstem stereotactic biopsy sampling in children. J Neurosurg 104(2): 108-114.
- Kuhn SA, Romeike B, Walter J, Kalff R, Reichart R (2009) Multiplanar MRI-CT fusion neuronavigation-guided serial stereotactic biopsy of human brain tumors: proof of a strong correlation between tumor imaging and histopathology by a new technical approach. J Cancer Res Clin Oncol 135(9): 1293-1302.
- Nemec SF, Donat MA, Mehrain S, Friedrich K (2007) CT–MR image data fusion for computer assisted navigated neurosurgery of temporal bone tumors. Eur J Radiol 62(2): 192-198.
- Prada F, Bene MD, Mattei L (2015) Preoperative magnetic resonance and intraoperative ultrasound fusion imaging for real-time neuronavigation in brain tumor surgery. Ultraschall Med 36(2): 174-186.
- Joud A, Stella I, Klein O (2020) Diffuse infiltrative pontine glioma biopsy in children with neuro navigation, frameless procedure: A single center experience of 10 cases. Neurochirurgie 66(5): 345-348.
- Larson PS, Starr PA, Martin AJ (2018) Deep brain stimulation: interventional and intraoperative mri approaches. Prog Neurol Surg 33: 187-197.
- Goda JS, Dutta D, Raut N (2013) Can multiparametric MRI and FDG-PET predict outcome in diffuse brainstem glioma? a report from a prospective phase-II Study. Pediatr Neurosurg 49(5): 274-281.
- Kossatz S, Carney B, Schweitzer M (2017) Biomarker-based pet imaging of diffuse intrinsic pontine glioma in mouse models. Cancer Res 77(8): 2112-2123.
- Huang TY, Piunti A, Lulla RR (2017) Detection of histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun 5(1): 28.
- Louis DN (2016) WHO pathology and genetics of tumours of the central nervous system. WHO Press pp. 57-59.
- Hoffman LM, Van Zanten S, Colditz N (2018) Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of Diffuse Intrinsic Pontine Glioma (DMG): a collaborative report from the international and European society for pediatric oncology dmg registries. J Clin Oncol 36(19): 1963-1972.
- Buczkowicz P, Hoeman C, Rakopoulos P (2014) Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46(5): 451-456.
- Nagaraja S, Vitanza NA, Woo PJ (2017) Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell 31(5): 635-652.
- Carvalho D, Taylor KR, Olaciregui NG (2019) ALK2 inhibitors display beneficial effects in preclinical models of ACVR1 mutant diffuse intrinsic pontine glioma. Commun Biol 2: 156.
- Baugh J, Bartels U, Leach J (2017) The international diffuse intrinsic pontine glioma registry: An infrastructure to accelerate collaborative research for an orphan disease. J Neurooncol 132(2): 323-331.
- Veldhuijzen ZSE, Baugh J, Chaney B (2017) Development of the SIOPE DMG network, registry and imaging repository: A collaborative effort to optimize research into a rare and lethal disease. J Neurooncol 132(2): 255-266.
- Li G, Mitra SS, Monje M (2012) Expression of epidermal growth factor variant III (EGFRvIII) in pediatric diffuse intrinsic pontine gliomas. J Neurooncol 108(3): 395-402.
- Mathew RK, Rutka JT (2018) Diffuse intrinsic pontine glioma: clinical features, molecular genetics, and novel targeted therapeutics. J Korean Neurosurg Soc 61(3): 343-351.
- Bredel M, Pollack IF, Hamilton RL, James CD (1999) Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res 5(7): 1786-1792.
- Fleischhack G, Massimino M, Warmuth MM (2019) Nimotuzumab and radiotherapy for treatment of newly diagnosed diffuse intrinsic pontine glioma (DIPG): a phase III clinical study. J Neurooncol 143(1): 107-113.
- Mehta AM, Sonabend AM, Bruce JN (2017) Convection-enhanced delivery. Neurotherapeutics 14(2): 358-371.
- Souweidane MM, Kramer K, Pandit TN (2018) Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol (8):1040-1050.
- Fonseca A, Solano P, Ramaswamy V (2021) Ventricular size determination and management of ventriculomegaly and hydrocephalus in patients with diffuse intrinsic pontine glioma: an institutional experience. J Neurosurg 5: 1-7.
- Guida L, Roux FE, Massimino M (2018) Safety and efficacy of endoscopic third ventriculostomy in diffuse intrinsic pontine glioma related hydrocephalus: a systematic review. World Neurosurg S1878-8750(18)32919-X.
© 2021 Reinas Rui. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






