- Submissions

Full Text
Techniques in Neurosurgery & Neurology
A Review of Aneurysmal Subarachnoid Hemorrhage Managed in Abuja, North-Central Nigeria
Ugwuanyi CU1*, Anigbo AA1, Nwaribe EE1, Ayogu OM1, Okpata CI1, Salawu MM2, Solanke O2 and Jamgbadi SS2
1Neurosurgery unit, National Hospital Abuja and Wellington Neurosurgery Center Abuja, Nigeria
2Neuroanasthesia unit, National Hospital Abuja and Wellington Neurosurgery center Abuja, Nigeria
*Corresponding author: Ugwuanyi CU, Neurosurgery unit, National Hospital Abuja and Wellington Neurosurgery Center Abuja, Nigeria
Submission: January 4, 2021;Published: April 15, 2021

ISSN 2637-7748
Volume4 Issue2
Abstract
Presentation of aneurysmal subarachnoid hemorrhage is often dramatic, and the management is even more challenging especially in a resource poor setting. There is therefore not a lot of local experience in the contemporary management of this inherently fatal form of stroke. This article shares our local experience in managing this condition in a resource constrained environment.
Aims and objectives: To report a four years’ experience in managing aneurysmal subarachnoid hemorrhage in Abuja, North Central Nigeria.
Methodology: Retrospective review of all cases that presented with CT confirmed aneurysmal subarachnoid hemorrhage from Jan 2016 to Jan 2020. Those with typical history of SAH, but unable to conduct these investigations were excluded. The biodata, clinical process, management and outcome were reviewed. Data generated were analyzed with simple descriptive statistics and presented accordingly.
Result: Only 27 (47%) patients out of 57 were sampled. 11 males and 16 females (M: F= 0.69: 1). Age range 26 -68 years. Mean age 46.4 years (SD=10.9). The shortest time to presentation was 4 hours, longest time to presentation was 168 days (Mean 26 days, SD 53.9 days). The commonest presenting complaints were headache 23/27 (85.1%), impairment of consciousness 14/27 (51.8%), Seizure 9/27 (33%). WFNS Grade 1 was the commonest presentation 12/27 (44%). The commonest observed risk factor was systemic hypertension in 18/27 (66.6%). The average mean arterial blood pressure was 115 mmHg, range 83.3-183, SD-26.9mmHg. Commonest was PCOM aneurysm 9/27 (33%) followed ACOM aneurysm 8/27 (29.6%). Intraventricular hemorrhage and associated acute obstructive hydrocephalus were observed in 12/27(44.4) requiring initial external ventricular drain EVD in 7/12 (58.3%) cases. The commonest operation was craniotomy for clipping in 15/27 (55.5%) followed by endovascular coiling in 3/27 (11.1%) Four (14.1%) rebled and died while awaiting definitive treatment, two (7.4%) are still awaiting treatments. Common surgical complications were extradural hematoma 4/15 (26.6%), Delayed ischemic neurological deficit (vasospasm) was observed in 3/27 (11.1%) especially in the post-surgical group. Rebleed was perhaps the most important because it caused a fatality. In one case hemiparesis and in another transient aphasia were recorded. Modified Rankin score was optimal in endovascularly coiled cases and in 13/27 (86.6%) of surgically clipped. 1/15(6.6%) died 48hrs while another (6.6%) was mRs 4 and required sustained support. Three were found to be non-aneurysmal perhaps from venous peri-mesencephalic bleed while two were confirmed aneurysmal bleeds but presently economically constrained to fund their planned surgical clipping since more than a year.
Conclusion: The numbers are small, but the study demonstrated sheer determination to treat brain aneurysms despite the great constraints. The experience gathered therefrom provides a high pedestal to achieve more in a better enabled environment.
Keywords: Cerebral aneurysms; Subarachnoid hemorrhage; Surgical clipping; Coiling
Introduction
Experience in the management of aneurysmal spontaneous SAH in Nigeria, dates back to the pioneering works of Odeku et al more than five decades ago. Based on the prevailing clinical knowledge at the time, in spite vigorous search by angiographic study of many suspected cases, he was surprised that contrary to expectation, the problem was not so prominent in comparison to figures from Europe and America [1]. Subsequent studies did not deviate much from this. For instance, data from a South Western Nigerian stroke registry revealed that 11.3% of strokes over a two-year period were due to subarachnoid hemorrhage [2]. And, from Enugu, South East Nigeria, 22 patients were managed as subarachnoid hemorrhage representing 58% of the hemorrhagic stroke patients between the periods of 1980- 1999 [3]. In Abuja, north central Nigeria, there is a concerted but unpublished effort by very few centers to match the increasing challenge of this potentially fatal disease condition. The aim of this paper is to expose the experience we have gathered in the last four years, articulate them and present in this report to hopefully add to the body of knowledge and improve practice.
Methodology
Ethical clearance was sought for and obtained from the three institutions involved in this study namely National Hospital Abuja, Federal Medical Centre Abuja and Wellington Neurosurgical Centre Abuja. Retrospective review of all cases that presented with CT confirmed aneurysmal subarachnoid hemorrhage from Jan 2016 to Jan 2020 was conducted. Those with typical history of SAH, but unable to conduct these investigations were excluded. The Biodata, clinical process (admitting neurology, Grading, CTA findings, Surgical treatment, endovascular treatment, complications of treatment), post op CT/CTA (Figures 1-3) and outcome were reviewed. Data generated were analyzed with simple descriptive statistics and presented accordingly.
Figure 1: Plain CT showing SAH. CTA showing culpable ACOM Aneurysm.
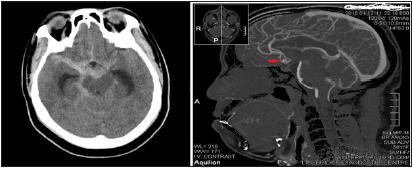
Figure 2:Plain skull Xray and CT showing titanium aneurysm clip in place post op.
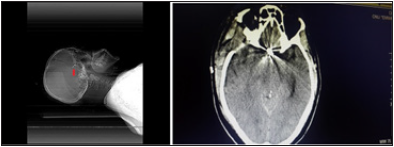
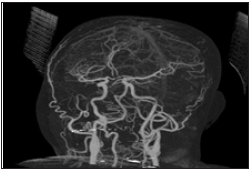
Figure 3:Post-op CTA showing satisfactory distal circulation after ACOM aneurysm clip application.
Result
27(47%) patients who presented to our services in Abuja, North Central Nigeria during the study period were recruited for this study. Our neurovascular services provide coverage for three tertiary institutions including National Hospital Abuja (three cases), Federal Medical Centre Abuja (one case) and Wellington Neurosurgery Centre Abuja (23 cases). There were 11 males and 16 females (M: F= 0.69: 1). The youngest patient was 26 years, the oldest was 68 years, mean age was 46.4 years (SD=10.9). The shortest time to presentation was 4 hours, longest time to presentation was 168 days (Mean 26 days, SD 53.9 days). The commonest presenting complaints were headache 23/27 (85.1%), impairment of consciousness 14/27 (51.8%), Seizure 9/27 (33%), vomiting 8/27 (29.6%) and others as listed in Table 1. WFNS Grade 1 was the commonest presentation 12/27 (44%) Table 2. The commonest observed risk factor was systemic hypertension in 18/27 (66.6%). Only one patient each had diabetes, renal disease, and retroviral disease. The average mean arterial blood pressure was 115mmHg, range 83.3-183, SD-26.9mmHg. Following relevant radiological evaluations including CT angiography, the commonly observed aneurysms were on the posterior communicating artery PCOM 9/27 (33%) and anterior communicating artery ACOM 8/27 (29.6%). Also observed were on the middle cerebral artery MCA 2/27 (7.4%), ophthalmic segment artery OSA 2/27(7.4) and anterior choroidal AChoA 1/27 (3.8).
Table 1:Presenting complaints and findings.
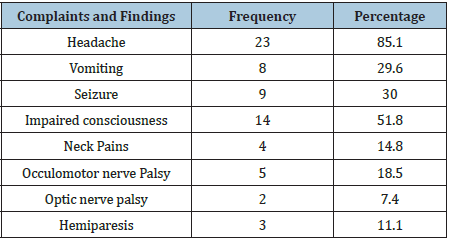
Table 2:WFNS grading.
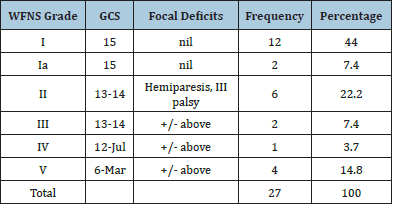
They were multiple in 2/27(7.4) and no aneurysm was
found in 2/27(7.4%) cases. Intraventricular hemorrhage and
associated acute obstructive hydrocephalus were observed in 12/27(44.4) requiring initial external ventricular drain EVD in
7/12 (58.3%) cases. The commonest definitive treatment modality
was craniotomy for clipping of identified aneurysm in 15/27
(55.5%) followed by endovascular coiling in 3/27 (11.1%) which
was usually outsourced. Whereas four (14.1%) rebled and died
while awaiting definitive treatment, five (18.5%) are still awaiting
treatment for reasons mostly related to financial constraints. The
initial EVD were subsequently converted to VP shunt when the
drainage became clear CSF. The commonest surgical approaches
were fronto-temporal and pterional craniotomy dictated by
the location of the aneurysm. For those who had surgery, the
following complications were observed: post op acute extradural
hematoma 4/15 (26.6%), Delayed ischemic neurological deficit
(DNID) manifesting as dropping GCS, aphasia in 3/15(20%).
Seizure was observed in one case but was adequately controlled
with phenytoin, a rebleed was observed from a second aneurysm
(superior hypophyseal aneurysm) which was not clipped at the
time of the initial craniotomy, this ultimately led to her demise. In
one case hemiparesis caused by infarct on the anterior limb of the
contralateral internal capsule was observed.
There was an injury to the posterior communicating artery
while dissecting and clipping the posterior communicating artery
aneurysm. In another case, transient aphasia, hemiparesis and
upper motor neuron facial nerve palsy was observed due to an
infarct on the head of caudate caused by most probably spasm
of the recurrent artery of Heubner during dissection of anterior
communicating artery aneurysm. All the endovascularly coiled and
treated cases were functioning optimally and reintegrated back
to their work and other pre-morbid engagements, while 13/15
(86.6%) of the craniotomy/clipped were able to attain this status.
1/15(6.6%) died 48hrs post-surgery from rebleeding and another
(6.6%) required sustained support for activities of daily living.
Three were found to be non-aneurysmal peri-mesencephalic bleeds
probably venous and responded well to neurocritical care, while
two were confirmed aneurysmal bleeds but severely economically
constrained to fund their planned surgical clipping since more than
a year.
Discussion
Only 27(47%) cases who presented to our services during the four years study period were sampled. The rest 53%, although presented with typical symptoms, and had initial diagnostic non contrasted CT but unable to proceed with CT Angio or indeed any further treatments, were not eligible for recruitment. It is also not possible that all the cases of SAH presented to our services as some could have made it elsewhere or even died before reaching hospital. Therefore, the numbers as observed in our series over this fouryear period most probably does not represent the actual prevalence in this environment. This account is only for those eligible for this study. The potentially fatal nature of this condition has been captured in previous reports that up to 15% die before reaching medical care, with an overall 14 days mortality of up to 50% in some series [4]. The lack of any decent insurance policy and heavy dependence on out-of-pocket payments and ‘family insurance’ for services are major factors that limit access to care.
It is expected that more of these conditions may be detected with improved registration of deaths and with more rigid policy on autopsy and notation of causes of death, particularly in young adults, in Nigeria. This dream was already put in place by the man considered as the father of Nigerian Neurosurgery Latunde Odeku since 1968 [1]. Most of this high-end neurosurgery care 23/27(85%) were concentrated on the private care facility here represented by Wellington Neurosurgery Centre Abuja, not necessarily because they have an overall higher capacity than the Government/Public hospitals but because they concentrate efforts to build capacity on such specialized areas whereas the latter is often saddled with enormous responsibility of provision of primary, secondary and tertiary care to a massive and expanding population and consequently innadequate attention is given to high end superspecialty care such as neuro, spine, cardiac, kidney transplant etc. This study showed that both sexes were fairly represented but with a slight preponderance towards the female sex (M: F= 0.69: 1) which is also in keeping with previous studies [5].
Although it was observed in the young as low as 26 years of age in this study it was by far commoner from the 4th decade of life with an average age incidence of 46years. This is also in agreement with other related findings that incident of SAH increases with age with an average onset after 50 years [6]. The delayed time to presentation (Mean 26 days, SD 53.9 days) in this study portrays poor response to initial management of this fatal disease condition in our environment. Delay in presentation and its relationship to poor outcome was already reported in previous and similar studies in this environment [1-3]. The reasons for delayed presentation are myriad but the recurring factor remains difficulty accessing decent neurosurgical services and poor understanding of the disease by the primary admitting hospital who often refer them at later stages when they deteriorate roundly. The most important complication of late presentation is re-bleeding from an untreated/ unsecured ruptured aneurysm. It has been reported that the maximal frequency of rebleeding is in the first day (4-13%) [7] and it has also been reported that up to 20% rebleed within the first two weeks and 50% within the first six months 8. Therefore, the often-late presentation in our environment encourages rebleeding and poor outcome. Headache often described as sudden and ‘worse ever’ remains the most consistent of all the presenting symptoms (85%) in this study as shown in Table 1 but may be up to 97% in some other series. This headache is often associated with coma due to increased intracranial pressure, and damage to brain tissue from expanding intracerebral hemorrhage, acute obstructive hydrocephalus from intraventricular bleed, seizures and also impairment of cerebral perfusion pressure [8,9].
Focal neurological deficits including hemiparesis, optic nerve and oculomotor nerve palsies were also observed. Optic nerve palsy was particularly observed in the case of ophthalmic segment aneurysm due to the close proximity in the optic canal. It manifested as ipsilateral complete blindness. Oculomotor palsy was found in the case of a posterior communicating artery aneurysm due to pressure on the adjacent oculomotor nerve. It manifested as ipsilateral ptosis, mydriasis and impaired extraocular muscles except the lateral rectus and superior oblique. Hemiparesis was due to acute hematoma dissecting into the brain parenchyma from a subarachnoid bleed and manifests as contralateral weakness. In agreement with other studies [10], commonest observed risk factor was systemic hypertension in 66.6% of cases with an average mean arterial blood pressure of 115mmHg, range 83.3- 183, SD-26.9mmHg. The observation also of diabetes, renal disease, and retroviral disease in a few cases were of uncertain link to the SAH. Chronic hypertension is known to cause endothelial injury, occlusion of the vasa vasorum, and disruption of the synthesis of collagen and elastin. These structural changes could initiate a focal weakening in the arterial wall with resultant bulging characteristic of aneurysms especially the saccular type 11. Following initial plain CT revealing a sub arachnoid bleed (Figure 1), all patients had CT angiography which revealed the culpable vessels/aneurysms as detailed in Table 3. In the absence of digital subtraction angiography (‘gold standard’), CT angiography has become the only available tool for investigating and treating neurovascular problems in our environment. In spite of its limitations, many centers have shown good results with CTA detecting up to 97% aneurysms [11,12].
Table 3: PCTA/Aneurysm by location.
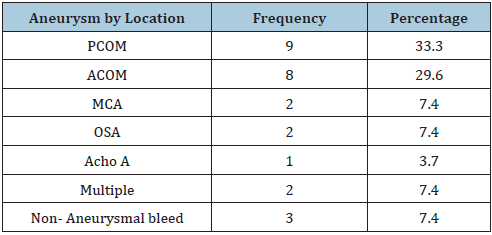
In situations of multiple aneurysms, it also has the capacity of differentiating the one that bled from others. Aside also providing a 3D image of neurovascular structures, CTA also demonstrates vital relationships to bony structures on the skull base which is useful for surgical planning. Table 3 confirms that posterior communicating aneurysm was the commonest (33.3%) but closely followed by anterior communicating artery aneurysm (29.6%). It also shows that nearly 93% of the aneurysmal SAH in this environment were situated on the anterior circulation. Our finding was in agreement with a similar study from East/Central Africa which demonstrated that posterior communicating artery was the most affected (35.7%), followed by the anterior communicating artery (26.8%), while the posterior cerebral artery was the least affected (2%) [13]. In some other studies especially from Caucasian background, the anterior communicating artery (ACOM) was found to be clearly in the lead with 34% but closely followed by MCA and the PCOM [14,15]. Other rarer types including ophthalmic segment, superior hypophyseal, and anterior choroidal aneurysms though rare were also identified. Of particular note were those where no identifiable aneurysm was seen after repeat CTA, suggesting a nonaneurysmal, prepontine, peri mesencephalic venous bleed. Both cases responded well to conservative treatments. Table 2 revealed that most of the treated cases (>80%) were good grade (WFNS III and above). This was because they presented way beyond the acute phase and were in much stable condition. Due to the absence of endovascular embolization services presently, majority of the patients 15/27 (55%) underwent open craniotomy and clipping of aneurysm. A few, 3/15 (11%) who had endovascular treatment were able to afford the huge logistics of outsourcing treatment overseas. But it is important to note that they were first stabilized by initial treatments until they were reasonably fit to embark on such. This underscores the need to continue the push towards evolving our practice towards providing endovascular treatment options as well. In spite of its much-debated methodological flaws, International Subarachnoid Aneurysm Trial (ISAT) contributed a lot to the current evidence base for decision making, patient counselling and consent taking in treating aneurysmal SAH.
Whereas 23.5% of 1063 patients allocated to endovascular treatment were dead or dependent at 1 year, it was up to 30.9% in the microsurgical arm, making a significant absolute risk reduction of 7.4% (95% CI 3.6-11.2, p=0.0001). This early survival advantage was maintained for up to 7 years and was considered significant (log rank p=0.03). The risk of epilepsy was substantially lower in patients allocated to endovascular treatment, but the risk of late rebleeding was higher. Therefore, although morbidity seems higher in microsurgical arm it clearly provides a more secure aneurysm neck and lowers the rate of re-bleeding. Other RCT studies such as the Barrow Ruptured Aneurysm (BRAT)Trial [16] adopted a stricter recruitment guideline which was considered all-inclusive without cherry picking and was able to prove that there was no statistically significant difference in modified Rankin Score (mRs) in the two arms but clearly favored microsurgery in terms of complete aneurysm obliteration at six years follow up especially for anterior circulation aneurysms. Based on the forgoing evidences, we were convinced that despite the endovascular treatment limitations in our environment, the available microsurgical treatment was safe reasonably dependable and was freely deployed. There was also no attempt to discourage any from seeking endovascular treatments overseas if they so desired. It is rather unfortunate that the lack of articulated insurance policy may have contributed to avoidable mortality and morbidity as four (14.1%) rebled and died while waiting to gather funds and logistics for surgery. And presently two are still awaiting funding for surgery with no clear hope in sight.
The surgical strategies deployed in managing intracranial aneurysms ranged from simple damage control procedure (emergency insertion of external ventricular drain-EVD) to a more elaborate craniotomy for microsurgical clipping. EVD was indicated in 7/27(25%) cases in this study to address the emerging issue of acute obstructive hydrocephalus consequent upon intraventricular extension of the subarachnoid bleed. It has been reported that severity of aneurysmal IVH is a strong contributor to initial severity and early complications of SAH [17]. It was also reported that large aneurysms, especially located in the anterior cerebral artery, are at particular risk of severe IVH and acute obstructive hydrocephalus [17]. Acute hydrocephalus worsens the already raised intracranial pressure with accelerated drop in GCS and instability in cardiorespiratory functions. This can prove potentially fatal if not quickly arrested. An initial EVD helps to arrest this descent, stabilize the patient and buy reasonable time to address the ruptured aneurysm. It is interesting to note that only three out of the seven initially treated with EVD, required long term CSF diversion via a VP shunt and the rest four were weaned off EVD successfully. Further CTA, confirmed the aneurysm site, size and numbers to dictate surgical planning. For a successful microsurgical treatment of intracranial aneurysms, aside the surgical expertise, certain equipment is mandatory including neurosurgical operating microscope, power drills, electrocautery bipolar system, suction machines, Rhoton’s micro-instruments and neurocritical care and radiology back up. Microsurgical approaches are unique for each type and location of the aneurysm but certain general principles for safe microdissection apply.
Hypotensive anesthesia, appropriate head position and fixation, judicious and careful brain retraction in a reasonably well relaxed brain, use of temporary clips when necessary, proper dissection of aneurysm neck, sound understanding of anatomy of aneurysm anatomy pre and post-dissection of the neck and of course proper placement of permanent clips in order to avoid compromise of any important vessels especially the perforators. In particular we have found brain relaxation key to a successful microdissection and minimal post op morbidity. The strategies adopted were sound anesthesia (sedation, with adequate ventilation and permissible hypotension), CSF drainage via lumbar drain, EVD insertion or intraop drainage of CSF from basal cisterns and other adjacent cisterns. In one case evacuation of associated ICH was done to achieve same. Anesthesia is key to a successful brain aneurysm repair and involve permissible hypotension and avoidance of blood pressure fluctuation. This involves suppression of laryngopressor response during endotracheal intubation, deepening analgesia during head fixation with Mayfield pins and also during skin incision and initial scalp dissection. Injection of local anesthetic at the Mayfield pin site and skin incision will avoid pain, thus reduce BP swings. It is also important to prevent cerebral ischemia by the ensuring of cerebral perfusion pressure at or above 70mmHg during the microdissection, brain retraction or clip application18. Aside surgical measures to achieve brain relaxation such as CSF drainage, anesthesia provides a key role by a combined use of propofol for maintenance, mannitol (0.5-1mg/kg) administered before dural opening, isoflurane above 1 minimum alveolar concentration to also improve perfusion.
Triple H therapy aimed at hypertension, hypervolemia and hemodilution has also been advocated to ensure smooth cerebral blood flow during and after aneurysm repair 19 but some consider it controversial. Communication between the surgical and anesthesia team is sustained all through the procedure and emerging issues are addressed with dispatch. Continuous multimodality monitoring includes but not limited to invasive BP monitoring, central venous pressure, capnography, urine output. Early and smooth emergence is proposed by some anesthetists in order to detect early and reversible complications of surgery [18,19]. But in our environment, there is a tendency to keep patients sedated and ventilated for at least 24hours post op before weaning and extubation.
PCOM artery aneurysm is the commonest encountered in this study, accounting for 33.3%. This is at variance with some other studies where it accounts for 15–25% of all intracranial aneurysm20 Commonly it arises at junction of Internal Carotid (IC) and Posterior Communicating Artery. (PCoA). According to the ISUIA study, PCoA aneurysm share natural history similar to other posterior circulation aneurysms in terms of higher five years cumulative risk of rupture (<7mm (2.5%), 7-12 mm (14.5%), 13-24 mm (18.4%), >25mm (50%)), compared to anterior circulation aneurysms 21. So, this study is rather in line with ISUIA study in terms of frequency of rupture as observed. The head is positioned and fixed on Mayfield pins such that the trajectory of microdissection remains perpendicular to the neck and dome of the aneurysm. Pterional craniotomy is usually preferred, and lesser sphenoid wing is drilled down towards the anterior clinoid process. This maneuver often creates unprecedented room that helps to minimize brain retraction. Following durotomy, most of the microdissection happens in the subarachnoid space. For PCOM aneurysm the key interest is to open the internal carotid and chiasmatic cisterns, to let out CSF and further relax the brain and most importantly to gain confidence of proximal parent artery control. Minimal brain retraction is done more on the frontal lobe than the temporal in order to prevent premature rupture of the often laterally pointing aneurysmal dome.
For the PCOM aneurysms, it has not become necessary to perform any anterior clinoidectomy in order to gain proximal purchase on the ICA because we almost always observed enough visual length and distance from the clinoidal segment of ICA to the neck of the aneurysm. In a related study with larger numbers though, a Korean group reported that only 6 out of 94 cases of PCOM aneurysm required anterior clinoidectomy [20-22]. So that’s rather lowish even in larger studies. It is mandatory to dissect the neck of the PCOM aneurysm off both the anterior choroidal artery and the posterior communicating artery and pack them off with gel foam to aid safe application of the suitably selected clip configurationstraight, bayoneted or curved depending on the anatomy of the aneurysm. A cross check is mandatory to ensure, only the intended aneurysm neck is clipped. The dome is then confidently aspirated and collapsed to improve visualization of the structures underneath and lateral especially the oculomotor nerve, and anterior choroidal and the rest of the posterior communicating artery. Meticulous hemostasis and water tight dural closure is achieved, bone flap replaced and wound closed in layers. For the lone case of anterior choroidal artery aneurysm, it was basically the same approach for the PCOM aneurysm. For the ACOM aneurysms, the approach is dictated by the size, morphology, associated ICH and direction of the aneurysm neck based on a thorough study of the 3D CTA. Commonly we adopt a more frontal fronto-temporal craniotomy. Extradural drill flattening of sphenoid wing and durotomy similar to above.
But the main subarachnoid microdissection focuses on the Internal
carotid and chiasmatic cisterns. Whereas the ipsilateral ICA
provides some proximal control, the aim is to expose the ICA ter minus, both A1s, A2s and of course the ACOM. Exposure of these
vessels improve the confidence of proximal control and can be enhanced
by evacuation of any associated hematoma, rectus gyrectomy
and opening of lamina terminalis to leak more CSF if required to
further relax the brain. The clip is carefully selected and applied but
avoiding all perforators and parent vessels especially the recurrent
artery of Heubner which is often seen running parallel to the A1.
Following successful clip application, the dome is punctured and
deflated to further remove pressure on adjacent structures.
Craniotomy access for the MCA aneurysm is essentially same as
above but more of temporal craniotomy access. The sub-arachnoid
space microdissection focuses on the splitting the Sylvian fissure
assisted by gentle medial temporal lobe and frontal lobe retraction
with not as much fear of premature rupture as in PCOM aneurysmal
dissection. It is standard in our procedure to first dissect the
proximal ICA for proximal control and gradually work towards the
terminus. Splitting the Sylvian fissure is commenced from the limen
insula and with the aid of frontal and temporal lobe retraction,
the whole length of M1 and M2 is exposed in accordance with the
dictates of the aneurysm location. Both cases in this series were
situated on the M2. The perforating lenticulostriate artery as well
as the bifurcating or trifurcating branches of the M2 terminus are
carefully dissected off the neck of the aneurysm before application
of appropriately selected clip is done. As usual the dome of the
aneurysm is aspirated and deflated.
For the two cases of ophthalmic artery aneurysm initial
dissection started from securing control by exposure of the ICA
in the neck. Craniotomy access was basically via pterional but
both extradural and intradural clinoidectomy were elaborate in
order to gain better access to the relevant surgical anatomy. It is
indeed the most difficult amongst this series because it was only
possible to apply clip on one while the other was wrapped with
a chunk of muscle harvested from temporalis as the best that can
be achieved. It was rather an unfortunate situation for one of the
cases of multiple aneurysm which was diagnosed in retrospect as
he rebled from the second initially undetected aneurysm (superior
hypophyseal) after the initial surgery secured ACOM aneurysm that
first bled. The second case was referred for endovascular treatment
of the second aneurysm on the ACOM after successfully clipping
the PCOM aneurysm. It is traditional to ventilate all overnight and
get a check CTA immediately upon weaning from sedation and
ventilatory support. Following complete cessation of sedation, GCS
and neurology in both upper and lower limbs are fully assessed
and documented. 3 D CTA also provides easy assessment of distal
flow. All this information is vital for further decision making. The
most important surgical complication in this series is one case of
intra-operative rupture representing 6.6%. It was experienced
during PCOM aneurysm repair. The application of both temporary
and permanent clips in a panic mode, though helped to arrest
the situation resulted in significant vascular compromise with a
resultant post op hemiparesis due to CT confirmed infarct on the
anterior limb of internal capsule. Reported rates of intra- operative
rupture of aneurysm range from 18-36% in pre-microscopic era 23
to 40% in recent studies [24] (Table 4).
Table 4:Definitive treatment.
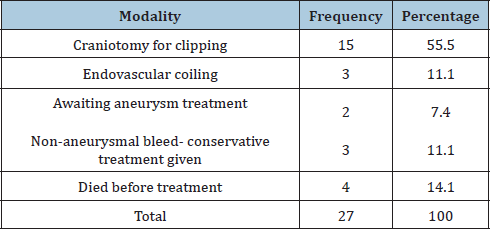
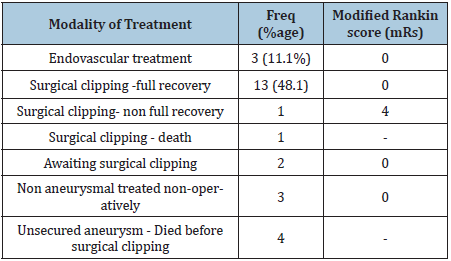
Table 5:Outcome of aneurysm treatment.
It has also been reported that morbidity and mortality increase
to 30-35% following this incident compared less than 10% when
there is no intra-operative rupture [25] for unclear reasons because
less is expected in the post- microscopic era. Rupture has been
reported during initial exposure, dissection of aneurysm or during
clip application but most occur during aneurysm dissection [26].
Therefore, due diligence is concentrated during those identified
stages of the operation in order to reduced and possibly eliminate
this unpleasant complication. Similarly, one of the patients with
ACOM aneurysm suffered transient aphasia and hemiparesis
due to compromise of the ipsilateral Heubner as evidenced by
infarct on the head of caudate on post op scan. Delayed Ischemic
Neurological Deficits (DNID) which was observed in three post op
patients resolved completely with adequate hydration and blood
pressure managements in line with the triple H therapy protocol.
Symptomatic vasospasm can occur in both surgically clipped
aneurysms (48%) as well as those treated endovascularly (43%)
but seems higher in the former and supported by studies by Li et al
27. Post op extradural hematoma requiring surgical evacuation was
observed in 4/15 (26%) but no additional morbidity consequent
upon this setback was recorded. Grandmal Seizure was recorded
in one case (6.6%) but responded well to standard loading and
maintenance dose of phenytoin. In general seizure complication
is not unusual but the incidence is quite low approximately 2%
in both treatment arms but slightly more in the surgical clipping
[23-29]. In terms of outcome (Table 5), it is interesting to report
that the two cases who still harbor unsecured aneurysms are still
fully functional independently and compliant with clinic follow
up till date. There have been no further incidences. Unfortunately, two confirmed cases are still awaiting surgery up to one year
after diagnosis due to financial constraints but remain in a sound
functional status. This is of major concern because their aneurysms
remain unsecured and the socioeconomic situation remain same
with absolutely no insurance policy to support. For sure, an
unsecured aneurysm that has previously bled remains a high risk
and uncertain when the rebleed may happen. There is however
some evidence that overall, 15-20% rebleed within 14 days, 50%
within 6 months, and thereafter the risk drops to 3% per year with
mortality of 2% per year [29].
Perhaps the mature gliotic and scar tissue around the aneurysm
dome have strengthened the previous weak point of the aneurysm
to withstand blood pressure fluctuations. It is also pertinent to note
that blood pressure management have remained satisfactory all
through their checkup periods. The high cost of brain aneurysm
surgery and the complete lack of any reasonable insurance policy
makes it almost impossible for poor patients to access needed
care. In complicity with human and material resource limitations,
this situation has tremendously affected the development and
expansion of neurovascular services in this environment. Therefore,
solving this hydra headed problem will definitely require a multifaceted
approach with a common denominator in funds. 86.6% of
the surgical clipped cases returned to full independent function
whereas all three endovascular treated cases remain stable and
have recorded consistent MRS zero and therefore able to return
to their normal activities and work. It is also important to report
that aside clinical stability, radiological checks with MRA have
not revealed any recurrence in previously treated aneurysm or
development of new aneurysms up to two years follow up.
Since the introduction of endovascular treatment of cerebral
aneurysms, there have been controversies over the superiority of
either arm but the ISAT and BRAT studies as outlined above have
been at the fore front of providing the necessary evidence to guide
treatment decision analysis and outcome. The key factor is that
treatment choice is guided by the realistic availability of resources.
For instance, in our practice, only open craniotomy for clipping is
available at the moment and that explains the much higher number
of surgical vs endovascular treated patients in this series. Only the
few with capacity to surmount the much higher financial forces of
overseas treatment, had endovascular treatment. Perhaps in the
future this trend may change with the evolution of endovascular
capacity locally.
Conclusion
There is an emerging neurovascular surgery practice in Abuja North central Nigeria but the figures presented in this report is rather not representative. This is because the practice environment is far from enabled and many cases disenfranchised. For now, PCOM Aneurysm tops the list but it is a work in progress. However, this study has demonstrated sheer determination by the authors with right attitude and culture laced with profound experience gathered in the past four years, to work around the current constraints and improve the outlook in the near future. There is therefore a high potential to achieve more in a better enabled environment to help more deserving patients.
References
- Odeku EL (1968) Intracranial vascular anomalies in Nigerians. J Natl Med Assoc 60(3): 173-180.
- Osuntokun BO, Bademosi O, Akinkugbe OO, Oyediran AO, Carlisle R (1979) Incidence of stroke in an african city: results from the stroke registry at Ibadan, Nigeria, 1973-1975. Stroke 10(2): 205-207.
- Onwuekwe, Nwabueze, Nwabueze, Aguwa (2008) The Challenge of subarachnoid haemorrhage in a regional teaching hospital in Nigeria. Journal of College of Medicine 13(1): 13-17.
- Sarti C, Tuomilehto J, Salomaa V (1991) Epidemiology of subarachnoid haemorrhage in Finland from 1983-1985. Stroke 22(7): 848-853.
- Rooij NK, Linn FH, Vander Plas JA, Algra A, Rinkel GJ (2007) Incidence of subarachnoid hemorrhage: a systematic review with emphasis on region, age, gender, and time trends. J Neurol Neurosurg Psychiatry 78(12): 1365-1372.
- Shea AM, Reed SD, Curtis LH, Alexander MJ, Villani JJ (2007) Characteristics of non-traumatic Subarachnoid heamorrhage in the United States in 2003. Neurosurgery 61(6): 1131-1137.
- Kassel NF, Turner J (1983) Aneurysmal rebleeding: a preliminary report from the co-operative aneurysm study. Neurosurgery 13(5): 479-481.
- Winn HR, Richardson AE, Jane JA (1997) The long-term prognosis in untreated cerebral aneurysm. The incidence of late hemorrhage in in cerebral aneurysm: A ten-year evaluation of 364 patients. Ann Neurol 1(4): 358-370.
- Ogilvy CS, Rordorf G, Bederson JB (1997) Mechanisms and treatment of coma after subarachnoid hemorrahege: Pathophysiology and management. American Association of Neurological Surgeons pp. 157-171.
- Wirth FP (1986) Surgical treatment of incidental intracranial aneurysms. Clin Neurosurgery 33: 125-135.
- Inci S, Spetzler RF (2000) Intracranial aneurysms and arterial hypertension: a review and hypothesis. Surgical Neurology 53(6): 530-540.
- Hoh BL, Cheung AC, Rabinov JD, Pryor JC, Carter BS (2004) Results of prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pre-treatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery 54(6): 1329-1340.
- Julius AO, Beda O, Justus Kilonzi, Simeon RS, Johnstone MM (2009) Intracranial aneurysms in an African country. Neurology 57(5): 613-616.
- Robert L. Dodd, Gary K (2003) Steinberg in Encyclopedia of the neurological sciences.
- Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, et al. (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366(9488):809-817.
- Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, et al. (2015) The barrow ruptured aneurysm trial 6-year results. J Neurosurg 123(3): 609-617.
- Darkwah OM, Gembruch O, Herten A, Frantsev R, Chihi M, et al. (2018) Intraventricular hemorrhage caused by subarachnoid hemorrhage: does the severity matter? World Neurosurg 111: e693-e702.
- Mylene L, Adrian WG (2015) Rev columbia of anasthesiology. Rev Columbia 43(1): 45-51.
- Elsayed MD, Anthony SW, Ehab F (2014) Anasthetic management of patients with intracranial aneurysms. Ochsner 4(3): 418-425.
- Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366(26): 2474-2482.
- Wiebers DO, Whisnant JP, Huston J III, Meissner I, Brown RD, et al. (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362(9378): 103-110.
- Park SK, Shin YS, Lim YC, Chung J (2009) Preoperative predictive value of the necessity for anterior clinoidectomy in posterior communicating artery aneurysm clipping. Neurosurgery 65(2): 281-285.
- Graf CJ, Nibbelink DW, Sahs Al (1981) Randomized treatment study: intracranial surgery. aneurysmal subarachnoid hemorhage. Report of the co-operative study, pp. 145-202.
- Pertusiet B, Pia HW, Langmaid C (1979) Intraoperative aneurysmal rupture and reduction by coagulation of the sac cerebral aneurysms- advances in diagnosis and therapy. Berlin Springer Verlag 398-128.
- SchrammJ, Cedzich C (1993) Outcome and management of intraoperative rupture of aneurysm. Surg Neurol 40(1): 26-30.
- Batjer H, Samson DS (1990) Management of Intraoperative Aneurysm Rupture. Clin Neurosurg 36: 275-288.
- Li H, Pan R, Wang H, Rong X, Yin Z, et al. (2013) Clipping Vs coiling for ruptured intracranial aneurysm: a systematic review and meta-analysis. Stroke 44(1): 29-37.
- Lanzino G, Durso PL, Suarez J (2011) Seizures and anticonvulsants after aneurysmal subarachnoid hemorrhage. Neurocrit Care 15(2): 247-256.
- Winn HR, Richardson AE, Jane JA (1977) The long-term prognosis in untreated cerebral aneurysms. The incidence of late hemorrhages in cerebral aneurysms: A 10-year evaluation of 364 patients. Ann Neurol 1(4): 358-370.
© 2021 Ugwuanyi CU. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






