- Submissions

Full Text
Techniques in Neurosurgery & Neurology
High Prevalence of Huntington’s Disease in CañetePerú
Torres L1*, Mori N2, Mazzetti P3, Mendoza4, Cuentas M4, Montoya J4, Mendoza H4, Domínguez J4, Pérez D4,Marca M5, Ortega O6 and Micheli F7
1Department of Neurodegenerative Diseases, Peru
2Department of Neurology, Peru
3Chief of the neurogenetics laboratory, Peru
4Physicians on Rotation, Peru
5Chemical Engineer at the Neurogenetics Laboratory at the Neurological Sciences Institute, Peru
6Biologist at the Neurogenetics Laboratory at the Neurological Sciences Institute, Peru
7Chief of the Parkinson’s Disease and Movement Disorders Program, Peru
*Corresponding author: Luis Torres Ramírez M, Department of neurodegenerative diseases, Peru
Submission: November 16, 2020;Published: December 16, 2020
ISSN 2637-7748
Volume3 Issue5
Abstract
Objective: To determine the prevalence of HD in five districts of Cañete Valley in order to develop a diagnosis, prevention and genetic counseling plan. Since HD is considered a hereditary disease with low prevalence, epidemiologic studies are scarce and lack genetic confirmation, which is nowadays necessary for the diagnosis of HD.
Methods: A first register of Cañete Valley inhabitants with HD was created in 1983. The population of this area has no access to health care or mass media, and the number of patients seeking for medical care is limited. Therefore, in 2004 we studied families systematically in five districts using the pedigree follow up method, which is ideal to determine the prevalence of genetic diseases and even more in communities like Cañete.
Results: We identified 30 genetically confirmed cases of HD (17 males, 13 females). The population of the five districts reached 66438 inhabitants on August 4th, 2004, i.e., a minimum prevalence of 45.1 per 100 000 inhabitants. We obtained 11 pedigrees, including 1397 individuals. Twenty-four (75%) patients were newly diagnosed cases of Huntington’s disease.
Conclusion: Cañete is the second largest focus of Huntington’s disease in Latin America, and one with the highest prevalence reported worldwide.
Keywords: Chorea; High prevalence; Huntington’s disease; Peru
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative condition, featuring complete penetrance, anticipation, with onset at middle age [1]. It is characterized by movement and psychiatric disorders as well as cognitive impairment [2,3]. Although this condition is widespread around the world, it is more prevalent in the Northern hemisphere, e.g., in the United States [4,5]. The HD gene in these countries was inherited from European immigrants. Isolated cases of HD have been reported in Latin America in the literature. Gatto et al. [6] published a clinical series including 11 Argentine patients with a diagnosis of HD whose main initial symptom was choreic-like movements. Cruz-Coke [7] in Chile reported 10 cases of HD, which together with the previous 22 cases reported in the Chilean literature totaled 32 cases in 12 families, 2 of these families with 10 cases were immigrants. Lima and Silva et al in Brazil [8] carried out genetic testing in 44 patients with HD and in a control group. Alonso [9] in Mexico reported his experience with 28 patients from 26 different families. Alfonso et al in Colombia, reported their experience including patients from some Colombian regions in 1995 [10,11].
No doubt, Maracaibo, in Zulia State, Venezuela is the hot spot for HD in Latin America. The number of patients with HD in this region represents the largest number of cases related to one common ancestor [12,13]. The high prevalence of HD in this region, which called the attention of the world medical community, was first reported by Negrette [13]. The first HD reports in the Cañete [14,15] Valley were unveiled by Cuba et al [16]; the prevalence for the Cañete province was then 31 cases per 100 000 inhabitants. In 1986 [17], the HD pedigree was studied, and Cañete was identified as the hot spot for the HD population in Peru. In 1989, Cuba [18] reported eight families with HD in Cañete, and in 1990 they reported 30 HD cases in one family only. This family was one of the 14 families assessed up to then. The authors concluded that the disease had appeared in that family 120-150 years before, to then spread from the Cañete Valley throughout Perú [19]. Learning about the prevalence of HD will lead to a better understanding of the condition in order to establish incidence rates and develop programs for improved diagnosis in Perú and more importantly, to provide adequate genetic counseling.
Methods
The pedigrees designed in 1983 were followed and updated [16,19]. A member of the family was interviewed in each case, and data were obtained to study the number of family members involved, age at the onset of symptoms, number of marriages and number of children. Once the new HD patients were identified in the new pedigrees, they underwent neurological examination. The prevalence was estimated considering the population census for the 5 districts in the Cañete Valley included in the previous study. (Quilmana, Pacaran, Imperial, Nuevo Imperial, Lunahuana) for August 2004. The districts and their populations are shown in Figure 1. Data for age, gender, age at symptom onset and family history were recorded. The patients underwent a clinical examination in their houses conducted by an experienced neurologist (L.T.R.) The diagnosis was made according to the clinical criteria proposed by Folstein [20]. It was later confirmed by means of molecular genetic studies once the patients had signed an informed consent to undergo blood sampling and participate in the study. Systematized neurological screening was also carried out, and the Unified Huntington's Disease Rating Scale (UHDRS) [21] used. The data were processed using the SPSS 12 statistical package for Windows. Frequency distribution, percentages, averages, and standard deviations were used to determine the magnitude and features of the study subject. The Student´s t test for non-matched samples was performed to determine significant differences, if any, as to the age of onset of the disease in relation to the parental inheritance pattern. p < 0.05 was considered a statistically significant value; the confidence interval was 95%.
Result
Frequency of the disease
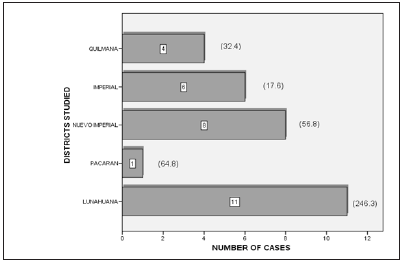
Eleven pedigrees were obtained including a total of 1397 individuals. Three new cases were found corresponding to two pedigrees previously studied by Cuba et al. [18] Further information was obtained for four generations for these cases. For the remaining 9 pedigrees information was obtained for at least five generations. Thirty patients were identified according to the pedigree follow up method. One patient, with a clinical diagnosis of HD from a family with genetic confirmation of the disease refused to participate in the study; therefore, this patient was not considered for the prevalence estimate. According to the National Institute of Statistics and Informatics and the population projections made for the years 1999-2005, the population in the 5 districts for the prevalence day (Figure 1), August 4, 2004, was 66438, which implies a minimum prevalence of 45.1/100 000 inhabitants. The cases per study area and their corresponding prevalence values are shown in Figure 2.
Figure 1: Canete valley and five districts of the study.
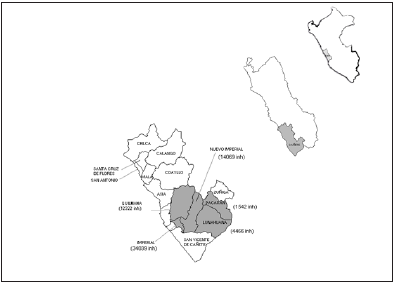
Figure 2: Huntington’s disease cases and prevalence according to districts studied.
Age and gender
All the patients were of mestizo origin, (mixed race Amerindian and white parentage origin), 17 (56.7%) were male and 13 (43.3%) were female: age range 23-71 yrs, mean age 43.2. The age and gender distribution are shown in Table 1. Of the 30 cases, 24 (75%) were first diagnosed by a neurologist.
Age of onset of clinical manifestations and time of the disease
The mean age of onset of clinical manifestations was 38.5 years (SD 14.3); the mean age for males was 36.8 years (SD 15.7) and, for females 40.6. (SD 12.6) (Table 2). In one patient (3.3%) onset of the disease occurred before the age of 20. The distribution of the stratified age of onset and gender is shown in Table 3. As for the inheritance pattern, 13(43.3%) patients reported having inherited the disease from their fathers, whereas 17(56.7%) inherited the condition from their mothers. No, de novo mutation was observed. The distribution of the age of onset depending on the parent involved appears in Table 4.
Table 1: Age and gender distribution at diagnosis.

Table 2: Age of onset according to sex.

aStatistical significance; SD: Standard Deviation.
Table 3: Age of onset by stratified groups according to sex [34].

Table 4: Relation between age at onset and sex of the affected parent.

*Number of cases aStatistical significance, SD: Standard Deviation
Clinical manifestations
Either the patients or their relatives were asked about three manifestations of the onset of the disease during the interview. Chorei-like movement disorders were present in 30 cases (100%), although some patients exhibited behavior disorders before or together with the chorei movements. Nine patients (30%) presented psychiatric disorders as the initial symptom; 8 of them had irritability, and one of them lacked motivation. None of these patients, even those with a late onset of the condition, presented dementia as the initial symptom. One patient (3.3%) developed the juvenile or early onset, though with rather atypical symptoms featuring involuntary movements involving the upper limbs and behavior disorders including irritability. In this case the patient had a paternal pattern of inheritance. One patient (3.3%) had memory impairment as the initial symptom.
Discussion
Table 5: HD prevalence around the world.

The prevalence of HD in the world ranges between 4 and 7/100 000 inhabitants [22]. Palo et al. [23] estimated the prevalence in Finland was 0.5 per 100 000 inhabitants, whereas in Western countries the prevalence ranges between 3 and 7 cases per 100 000 inhabitants. Incidence among the Japanese [24], South Africans [25] and African Americans [12] is the lowest. However, the prevalence of HD is over 15/100 000 cases in some countries, mainly in Western Europe [26]. The distribution of HD prevalence in different regions of the world is shown in Table 5. The prevalence in Lake Maracaibo, Venezuela, reaches 700 per 100 000 inhabitants [27]. According to the results obtained in this study, Cañete might be the second largest HD population in Latin America and one of the most important ones globally. The increase in prevalence is alarming when considering the data published by Cuba et al. [16]. Such a high prevalence in this area might be due to a combination of social and geographic isolation leading to the spread of this genetic inherited disorder, which occurs when the gene is introduced in a given population with a high growth rate [12]. Interestingly, 75% of the patients had no previous diagnosis of HD, which enhances the relevance of the methodology applied in this particular study, since in other scenarios where patients have access to health care centers and communication networks a different methodology may be used, even the recent capture and recapture method used by Burguera et al. [28] in Valencia, in which case it was necessary to cross several health care information sources [29,30]. Folstein [4] conducted a study in Maryland, and reported that the pedigree follow up method enabled a more accurate identification of cases among African Americans as compared to Caucasians; the latter had more access to both radio, television and health care centers.
According to Folstein et al [20], studies conducted in the community exhibit two main sources of diagnostic error: inaccurate research in the family history, and the lack of knowledge about clinical features and course of the disease. In our study, the diagnosis issue could be solved by means of systematic interviews to the patients’ relatives and a precise pedigree design; in all cases the disease had been genetically confirmed. Clinical manifestations typically include a phase characterized by mild behavioral and psychiatric disorders which develops up to 10 years before choric manifestations occur. Shiwach and Norbury [31] proved that psychiatric symptoms were common in HD before the occurrence of neurological symptoms. In our study, a third of the patients exhibited behavior disorders before or together with chorea.
All the patients in our study had typical HD, even the patient with juvenile or early onset of HD and the cases of late onset of the disease (3.3 and 23.3%, respectively) as compared to other series [32]. HD occurs at about the age of forty, which correlates with most of the results obtained in previous studies when considering juvenile, typical and late onset HD; whereas late onset HD occurs at about the age of fifty [33,34].
Conclusion
Based on the data obtained, Cañete is the second largest HD population in Latin America, and one of the largest in the world. It is vital to implement programs to provide counseling to HD patients and relatives at risk for this disease.
Acknowledgment
To Juan Manuel Cuba Rodríguez, Ángel Sagástegui Urteaga MD and Joanna Urrego for their friendship and counseling.
References
- Degenhardt L, Charlson F, Mathers B (2014) The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction 109(8): 1320-1333.
- Schuckit MA (2016) Treatment of opioid-use disorders. N Engl J Med 375(16): 1596-1597.
- Bell J (2014) Pharmacological maintenance treatments of opiate addiction. Br J Clin Pharmacol 77(2): 253-263.
- Gossop M, Marsden J, Stewart D (2001) Outcomes after methadone maintenance and methadone reduction treatments: two-year follow-up results from the national treatment outcome research study. Drug Alcohol Depend 62(3): 255-264.
- Bell J, Strang J (2020) Medication teatment of opioid use disorder. Biol Psychiatry 87(1): 82-88.
- Amato L, Minozzi S, Davoli M (2011) Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev 5(10): CD004147.
- Mattick RP, Breen C, Kimber J, Davoli M (2014) Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Systematic Rev 2: CD002209.
- Soyka M, Strehle J, Rehm J (2017) Six-year outcome of opioid maintenance treatment in heroin-dependent patients: results from a naturalistic study in a nationally representative sample. Eur Addict Res 23(2): 97-105.
- Volkow ND, Frieden TR, Hyde PS (2014) Medication assisted therapies: tackling the opioid-overdose epidemic. N Engl J Med 370(22): 2063-2066.
- Mattick RP, Breen C, Kimber J (2014) Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 3: CD002207.
- Lofwall MR, Walsh SL, Nunes EV (2018) Weekly and monthly subcutaneous buprenorhpine depot formulations vs daily sublingual burpenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med 178(6): 764-773.
- Bell J (2010) The global diversion of pharmaceutical drugs: opiate treatment and the diversion of pharmaceutical opiates: a clinician’s perspective. Addiction 105(9): 1531-1537.
- Fareed A, Vayalapalli S, Casarella J (2012) Effect of buprenorphine dose on treatment outcome. J Addict Dis 31(1): 8-18.
- Greenwald MK, Comer SD, Fiellin DA (2014) Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend 144: 1-11.
- Hser YI, Evans E, Huang D (2016) Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 111(4): 695-705.
- Jordan CJ, Newman AH, Xi ZX (2019) Progress in agonist therapy for substance use disorders: lessons learned from methadone and buprenorphine. Neuropharmacology 158: 107609.
- Walter M, Soyka M (2019) Opioide. In: Soyka M, Batra A, et al. (Eds.), Hrsg Suchtmedizin München, Elsevier, Netherlands, pp. 177-202.
- Deamer RL, Wilson DR, Clark DS (2001) Torsades de pointes associated with high dose levomethadyl acetate (ORLAAM). J Addict Dis 20(4): 7-14.
- Nasser AF, Heidbreder C, Gomeni R (2014) A population pharmacokinetic and pharmacodynamic modelling approach to support the clinical development of RBP-6000, a new, subcutaneously injectable, long-acting, sustained-release formulation of buprenorphine, for the treatment of opioid dependence. Clin Pharmacokinet 53(9): 813-824.
- Laffont CM, Gomeni R, Heidbreder C (2016) Population pharmacokinetic modeling after repeated administrations of rbp-6000, a new, subcutaneously injectable, long-acting, sustained-release formulation of buprenorphine, for the treatment of opioid use disorder. J Clin Pharmacol 56(7): 806-815.
- Haight BR, Learned SM, Laffont CM, Fudala J, Zhao Y, et al. (2019) Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 393(10173): 778-790.
- Ling W, Shoptaw S, Goodman M (2019) Depot buprenorphine in the management of opioid use disorder. from development to implementation. Subst Abuse Rehabil 10: 69-78.
- Coe MA, Lofwall MR, Walsh SL (2019) Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med 13(2): 93-193.
- Albayaty M, Linden M, Olsson H (2017) Pharmacokinetic evaluation of one-weekly and once-monthly buprenorphine subcutaneous injection depots (CAM 2038) versus intravenous and sublingual buprenorphine in healthy volunteers under naltrexone blockade: an open-label phase 1 study. Adv Ther 34(2): 560-575.
- Walsh SL, Comer SD, Lofwall MR (2017) Effect of buprenorphine weekly depot (CAM 2038) and hydromorphone blockade in individuals with opioid use disorder: a randomised clinical trial. Jama Psychiatry 74(9): 894-902.
- Frost M, Bailey GL, Lintzeris L (2019) Long-term safety of weekly and monthly subcutaneous Buprenorphindepot (CAM 2038) in the treatment of adult out-patients with opiod use disorders. Addiction 114(8): 1416-1426.
- Barnwal P, Das S, Mondal S (2017) Probuphine® (buprenorphine implant): a promising candidate in opioid dependence. Ther Adv Psychopharmacol 7(3): 119-134.
- Itzoe M, Guarnieri M (2017) New developments in managing opioid addiction: impact of a subdermal buprenorphine implant. Drug Des Devel Ther 11: 1429-1437.
- White J, Bell J, Saunders JB (2009) Open-label dose-finding trial of buprenorphine implants (Probuphine) for treatment of heroin dependence. Drug Alcohol Depend 103(1-2): 37-43.
- Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, et al. (2010) Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA 304(14): 1576-1583.
- Rosenthal RN, Ling W, Casadonte P (2013) Buprenorphine implants for treatment of opioid dependence: randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction 108(12): 2141-2149.
- Crotty K, Freedman K, Kampman K (2020) Executive summary of the focused update of the asam national practice guideline for the treatment of opioid use disorder. J Addict Med 14(2): 99-112.
- Pendergrass SA, Crist RC, Jones LK, Hoch JR, Berrettini WH (2019) The Importance of buprenorphine research in the opioid crisis. Mol Psychiatry 24(5): 626-632.
- Kelty E, Cumming C, Troeung L, Hulse G (2018) Buprenorphine alone or with naloxone: which is safer? J Psychopharmacol 32(3): 344-352.
- Timko C, Schultz NR, Cucciare MA (2016) Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis 35(1): 22-35.
- Allikmets S, Vink JP (2020) Clinical applications of burprenorphine depot injection for opioid use disorder. Addiction 115(1): 190.
- Soyka M (2020) Novel long-acting buprenorphine medications for opioid dependence: current update. Pharmacopsychiatry 53.
- Vorspan F, Hjelström P, Simon N, Benyamina A, Dervaux A, et al. (2019) What place for prolonged-release buprenorphine depot-formulation buvidal in the treatment arsenal if opioid dependence? insights from the french experience on buprenorphine. Expert Opin Drug Deliv 16(9): 907-914.
- Kenney SR, Anderson BJ, Bailey GL (2018) Buprenorphine treatment formulations: preferences among persons in opioid withdrawal management. J Subst Abuse Treat 94: 55-59.
- Larance B, Degenhardt L, Grebely J, Nielsen S, Bruno R, et al. (2019) Perceptions of extended-release buprenorphine injections for opioid use disorder among people who regularly use opioids in Australia. Addiction 115(7): 1295-1305.
- Neale J, Tompkins CNE, Strang J (2019) Prolonged-release opioid agonist therapy: qualitative study exploring patients views of 1-week, 1-month and 6-month buprenorphine formulations. Harm Reduct J 16(1): 25.
- Tompkins CNE, Neale J, Strang J (2019) Opioid users’ willingness to receive prolonged-release buprenorphine depot injections for opioid use disorder. J Subst Abuse Treatment 104: 64-71.
- Atzendorf J, Rauschert L, Seitz NN (2019) Gebrauch von alkohol, tabak, illegalen drogen und medikamenten. Dtsch Ärzteblatt 116: 577-584.
- Bell J, Trinh L, Butler B (2009) Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction 104(7): 1193-1200.
- Connor AM, Cousins G, Durand L, Barry J, Boland F (2020) Retention of patients in opioid substitution treatment: a systematic review. Plos One 15(5): 0232086.
- European Monitoring Centre for drugs and Drug addiction (2020) European Drug Report.
- Greenwald MK, Johanson CE, Moody DE (2003) Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology 28(11): 2000-2009.
- Haasen C, Linden M, Tiberg F (2017) Pharmacokinetics and pharmacodynamics of a buprenorphine subcutaneous depot formulation (CAM2038) for once-weekly dosing in patients with opioid disorder. J Subst Abuse Treat 78: 22-29.
- Hser YI, Saxon AJ, Huang D (2014) Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction 109(1): 8-18.
- Kimber J, Larney S, Hickman M (2015) Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiat 2(10): 901-908.
- Rosenthal RN, Lofwall MR, Kim S (2016) Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: a randomized clinical trial. JAMA 316(3): 282-290.
- Strang J, Groshkova T, Uchtenhagen (2015) Heroin on trial: Systematic review and meta-analysis of randomised trials of diamorphine prescribing as treatment for refractory heroin addiction. Br J Psychiatry 207(1): 5-14.
- United Nations Office on Drugs and Crime (2017) World drug report. vienna: united nations office on drugs and crime.
- United Nations Office on Drugs and Crime (2020) International standards on drug use prevention second updated edition.
- Wieneke H, Conrads H, Wolstein J (2009) Levo-alpha-acetylmethadol (LAAM) induced QTc-prolongation-results from a controlled clinical trial. Eur J Med Res 14(1): 7-12.
© 2020 Michael Soyka. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






