- Submissions

Full Text
Techniques in Neurosurgery & Neurology
An Artificial Neural Network Predicts the Excessive Radiation Risk in Epidural Pulsed Radiofrequency (Eprf) Interventions- A Pilot Study
Georgios Matis*
Department of Stereotactic and Functional Neurosurgery, University Hospital Cologne, Germany
*Corresponding author: Georgios Matis, Department of Stereotactic and Functional Neurosurgery, University Hospital Cologne, Germany
Submission: November 15, 2019;Published: January 21, 2020

ISSN 2637-7748
Volume3 Issue2
Abstract
Objectives: Epidural pulsed radiofrequency (ePRF) interventions are successfully used in the treatment of patients with cervical, thoracic, and lumbar pain but they can be associated with high radiation doses. The scope of this study was to evaluate the ability of an artificial neural network (ANN) to predict preoperatively an excessive radiation risk in such procedures.
Materials and Methods: For 46 patients treated with ePRF, the dose area product (DAP) and procedure times were retrospectively analyzed. Additional patient, symptom, and surgical characteristics were collected based on the surgery protocols. An ANN was constructed to predict the excessive radiation as compared to the mean DAP value.
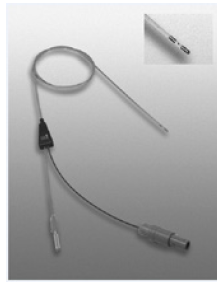
Results: Twenty-one patients were male (45.7%) and 25 females (54.3%). Mean values and ranges: age (61.76±2.2; 29-86 years), duration (26.65±1.43; 12-53 minutes), and DAP (694.63±113.83; 130.71- 3,711.64 Gy/cm2). The resulted ANN contained 7 scaling neurons (inputs), 3 hidden neurons, and one probabilistic neuron (target). Important inputs for the acquired ANN were age, sex, diagnosis, side, and level of intervention. The experience of the surgeon and the duration of the surgery were not significant contributors in this ANN. The network exhibited a sensitivity of 1, a specificity of 0.43, an AUC (Area Under Curve, ROC chart) of 0.714.
Conclusion: It was possible to construct an ANN, which could predict if the radiation burden during an ePRF procedure would be higher than the average one. This could prove useful in optimizing the planning of ePRF procedures. Further studies with a larger series of patients are warranted.
Keywords: Artificial neural networks; Interventions; Prediction; Pulsed radiofrequency; Radiation risk.
Introduction
Pulsed radiofrequency (PRF) interventions are characterized as minimally invasive and target-selective procedures reducing the pain intensity in various chronic pain syndromes [1,2]. Many studies have demonstrated the effectiveness of this therapy [3-5]. Surgeons find it simple, easy and with a low risk profile [5,6]. Nevertheless, PRF surgeries are associated with specific risks too. The use of fluoroscopy can pose radiation risks not only for the patient but for the surgeon as well [7]. Health professionals working with ionizing radiation should always take into consideration the radiation dose they and their patients may receive during an interventional procedure and try, by using a careful preoperative planning, to eliminate or reduce the factors, which determine the level of these doses [8]. Artificial neural networks (ANNs), as computing systems inspired by biological neural networks, incorporate the technique of memorizing heterogeneous correlations between input and output patterns [9]. After a training phase, they can extrapolate to give answers to newly presented input data [9-11]. ANNs have been used since many years successfully for predicting outcomes in various medical fields [11-16]. Interestingly, up to this point, Neuromodulation scientists have conducted extremely limited research in this promising evolving domain [17-19]. The existing publications are based mostly on preclinical settings [20,21]. The scope of this pilot study was to generate initial observations on the feasibility of the ANN-technology to predict preoperatively an increased radiation risk in patients, who undergo ePRF interventions.
Materials and Methods
Patient population
A total number of 46 consecutive patients with chronic neuropathic pain (P) treated in our Department during the last 6 months with ePRF were enrolled (Table 1). The dose-area product (DAP) [22] and the procedure times were retrospectively analyzed. Additional patient (age, sex), symptom (low back-P, leg-P, back and leg-P, thoracic-P, cervical-P, left or right side), and surgical (experience of surgeons, level of intervention) characteristics were collected based on the surgery protocols. Most of the patients were female (54.3%), and the mean age was 61.76 years. The mean duration of the intervention was 26.65 minutes, and the mean DAP was 694.63Gycm2). The most frequent symptom was the combination of low back- and leg-P (52.2%). Pain was manifested more often on the right side (43.5%). The PASHA catheter was placed in the majority of the cases at the conus medullaris level (93.5%). Surgeons with an experience of 2-5 years performed 56.5% of the surgeries.
Table 1: Patient, surgeon, and surgery characteristics.
ePRF-Technique
The tip of the multifunctional PASHA-Catheter [23] (Figure 1) was placed in the epidural space by typical epidural punction under fluoroscopy directly to the target root or at the level of conus medullaris. A motor stimulation at 2Hz was made followed by a sensory one at 75Hz, evoking a paresthesia in the painful area. When the expected response was observed, we applied the PRF for 4-12minutes (42 oC). The radiofrequency current was applied in short (20ms) and high-voltage bursts with a silent phase of 480ms, which allowed time for heat elimination. At the same time, we injected 40mg Dexamethasone (acis Arzneimittel GmbH, Gruenwald, Germany), and 6ml Ropivacaine 0,5% (AstraZeneca GmbH, Wedel, Germany).
Figure 1: PASHA-Catheter used to inject drugs and provide ePRF [23].
ANN
An advanced analytics software (Neural Designer, Artificial Intelligence Techniques Ltd., Salamanca, Spain) was used to construct an ANN based on 7 input (age, sex, diagnosis, side, level of surgery, experience of surgeon, duration of the surgery) and 1 target (excessive radiation as compared to the mean DAP value from all patients, DAP OVER MEAN) variables. Nine patients were excluded from the analysis because of missing data. Variables from 21 patients were analyzed during the training phase (quasi- Newton training algorithm). The quasi-Newton method is based on Newton’s method but does not require calculation of second derivatives. Instead, it computes an approximation of the inverse Hessian at each iteration of the algorithm, by only using gradient information [24]. Eight patients were included in the testing phase. The ANN was then used to predict the excessive radiation risk in 8 patients (validating phase).
Receiver operating characteristic (ROC) curve
The discrimination capacity of the ANN was tested with the ROC curve. A ROC curve is a graph, which plots in the x-axis the 1.0-specificity and in the y-axis the sensitivity calculated for every different threshold [25]. In order to calculate the points of the curve, the threshold varies along the output probability of the testing instances in ascending order. In a perfect model, that can correctly classify all the instances, the ROC curve passes through the upper left corner, which is the point in which the sensitivity and specificity take the value 1. The closer to the upper left corner that the ROC curve passes, the better its discrimination capacity (the area under curve, AUC, for it would be 1) [26]. A random classifier, represented by the base line, has an AUC of 0.5 [11].
Statistical analyses
All analyses (mean, standard deviation, range, ROC-curve, AUC) were conducted using SPSS, Version 17.0 (SPSS Inc., Chicago, IL, USA) and Neural Designer (Artificial Intelligence Techniques Ltd., Salamanca, Spain).
Results
Table 2: Inputs importance results for the artificial neural network (ANN).
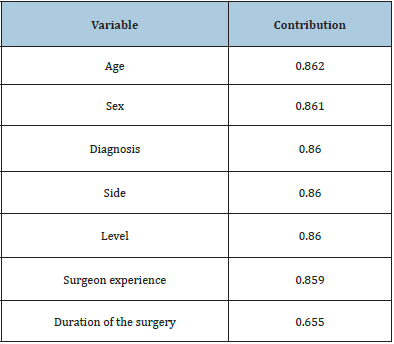
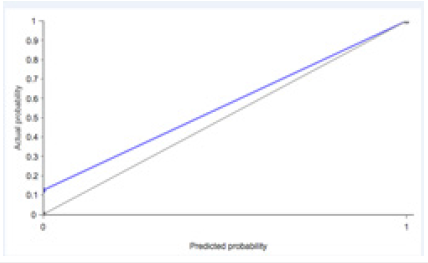
The graphical representation of the network architecture is depicted in Figure 2. The resulted ANN contained 7 scaling neurons (inputs), 3 hidden neurons (representative of the complexity of the ANN) and one probabilistic neuron (target; DAP OVER MEAN = yes / no). Important inputs for the acquired ANN were age, sex, diagnosis, side, and level. Table 2 shows the importance of each input. The most important variable was Age, which got a contribution of 86.22% to the outputs. The experience of the surgeon and the duration of the surgery were not significant contributors in this ANN. The network exhibited a sensitivity of 1, a specificity of 0.43, an AUC of 0.714 (Figure 3), and a maximum gain score (maximum difference between the positive cumulative gain and the negative cumulative gain, i.e., the point where the percentage of positive instances found is maximized and the percentage of negative instances found is minimized) of 0.857 (in a perfect model = 1) (Figure 4). The calibration plot (Figure 5) showed a good calibration too.
Figure 2: Neural network graph. The ANN contains a scaling layer, a neural network and a probabilistic layer. The yellow circles represent scaling neurons, the blue circles perceptron neurons and the red circles probabilistic neurons. The complexity, represented by the numbers of hidden neurons, is 3.

Figure 3:Receiver Operating Characteristic (ROC) curve.
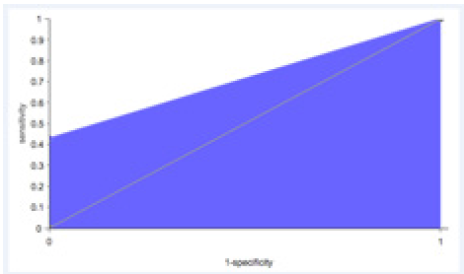
Figure 4:Cumulative gain plot. The blue line represents the positive cumulative gain, the red line represents the negative cumulative gain and the grey line represents the cumulative gain for a random classifier. The more separation between the positive and negatives cumulative gain charts, the better the classifier.
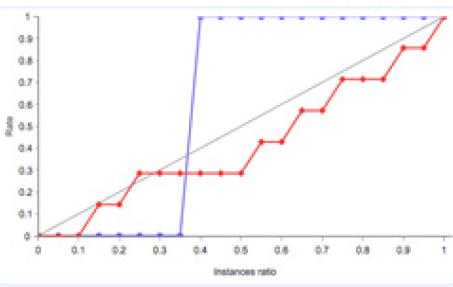
Figure 5:Calibration chart. It represents the true probability versus the estimated probability. The base line represents a classifier with a perfect calibration.
Discussion
The goal of the study was to investigate the potential applicability of the ANN-technology in predicting an excessive radiation risk in ePRF patients. It is, to the best of our knowledge, the first identifiable article in the literature, which investigates the possible role of such constructs in improving the quality of surgeries in the epidural space. PRF represents a Neuromodulation therapy that is effective in the context of neuropathic pain [27]. It has emerged from an Austrian conference in 1995 [1,28], but the first procedure was performed on February 1st, 1996 [5]. It offers pain reduction without causing destructive lesions [29,30]. Although the mechanism of action has not been fully understood, laboratory experiments show real neurobiological processes modulating the pain signaling [3]. It has been used in treating various conditions, such as cervical [4,31,32] and lumbar radicular pain [4,31,33], sacroiliac and shoulder joint pain [34]. Table 3 presents some indicative studies investigating the efficacy of this modality [4,31-34]. Unfortunately, PRF requires fluoroscopy, which in turn is associated with exposure to hazardous ionizing radiation [35]. The DAP assesses the occupational dose and provides valuable information about the extent of the scatter dose and the appropriateness of the applied techniques [22]. It was first in 1992 that the Food and Drug Administration (FDA, USA) received some unverifiable reports linking the use of fluoroscopy to possible radiation injuries [36]. Since then, the issue of radiation exposure has been extensively addressed during different types of diagnostic procedures and surgeries, such as in lumbar discography [37-40], in vertebroplasty [39,40], in kyphoplasty [41], in minimally invasive transforaminal lumbar interbody fusion [42], and in pedicle screw insertion in the lumbar spine [43-45]. In pain practice, the radiation levels were explored during lumbar epidural steroid injections [38,46-52], in medial branch blocks and facet joint injections [38,50-54], in intercostal blocks [51], in stellate ganglion blocks [55], and in percutaneous adhesiolysis [51].
Table 3: Indicative publications on pulsed radiofrequency (PRF) [4,31-34].
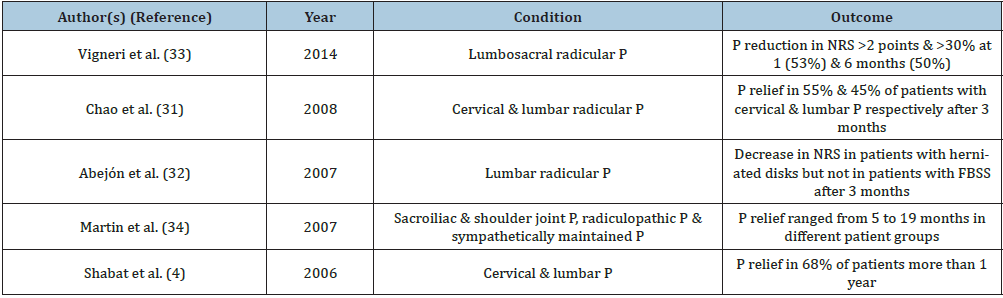
FBSS: Failed Back Surgery Syndrome; NRS: Numeric Rating Scale; P: Pain
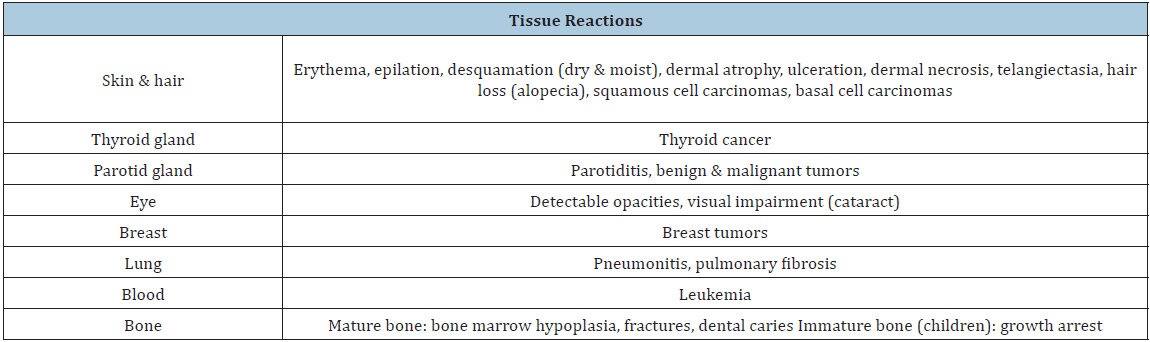
Potential biological effects following interventional procedures are manifested in the skin [36,56-58], in the eyes [58-60], in glands (thyroid, parotid) [58], and in several other tissues (breast, lung, bone, blood) [58,61-63]. In contrast to the above findings, the occupational exposure to X-rays seems unlikely to be associated with congenital abnormalities or childhood malignancies in the practitioners’ off springs [64]. An overview of the reported health risks due to radiation is provided in Table 4. Thus, proactively reducing the radiation risk is necessary to ensure the safety of surgeons, patients, and personnel as well [35,54,65]. The preprocedural planning should incorporate the evaluation of radiation risk to the patient considering all the relevant demographic, medical, and procedural risk factors [66]. Radiation reducing methods include, among others, minimization of beam-on time, minimization of the use of image intensifier magnification, proper collimation of the primary beam, additional beam filtration, lower dose-mode fluoroscopy, lower recording speed, higher X-ray tube potential, and image receptor as close to patient as possible [67]. Our study found that an increased radiation risk (DAP OVER MEAN) was noted in 21.6% of the surgeries, and this risk was correlated with age, sex, side, diagnosis, and level of surgery. Age was the most significant contributor. The existing literature provides no information about the role of age, sex, and side of surgery on the radiation exposure. The correlation of the exact pain cause (diagnosis) is also poorly studied. More data are accumulated about the level of surgery. For instance, Hwang et al. compared the radiation exposure during transforaminal fluoroscopy-guided epidural steroid injections (TFESIs) at different vertebral levels [49]. Fluoroscopy time was significantly shorter at L5 than that at L2-L4 level (p=0.004). DAP at L5 and S1 levels were also significantly lower than that at L2- L4 levels. After correcting for fluoroscopy time, DAP was found to be significantly lower at S1 than at either L2-L4 (p=0.001) or L5 (p=0.010) levels. Another study evaluated the radiation exposure to the spinal interventionalist performing lumbar discography [37]. On discograms at the L5/S1 level, a significantly higher radiation exposure was measured as compared to the L3/L4 (p=0.008) and the L4/L5 levels (0.009). We performed only 2.2% of the interventions at the cervical level, and 4.3% at the middle thoracic level. In this population, the main target was in 93.5% of the patients the conus medullaris. Its position was preoperatively confirmed on midline T2-weighted sagittal magnetic resonance imaging (MRI) studies.
Table 4: Potential effects of radiation exposure.
Only 19.6% of the surgeries were performed by a surgeon with an experience of >5 years and 23.9% by a surgeon with an experience of <2 years. The experience of the surgeon was not a significant contributor in our ANN, although the literature provides evidence that the interventionalist experience constitutes a major factor [54,68-71]. It has been documented that residents who perform spine surgeries receive more radiation in comparison to residents who perform other interventional procedures [68]. Fluoroscopy times and patient doses were found to be reduced in the presence of experienced physicians during cerebral and lower limb diagnostic procedures [69], and during endoscopic retrograde cholangiopancreatography (ERCP) [70]. In addition, the presence of a coach, who gave the pain physicians instructions to minimize their received scatter dose during 596 interventional pain procedures, achieved a significant dose reduction of 46.4% [54]. Another study of 402 endourological interventions showed that surgeons reduced their fluoroscopy times up to 55% after receiving information/advice about their fluoroscopy manners. This finding was valid only for experienced surgeons and for easy procedures [71]. In contrast to these studies comes a report on patients undergoing coronary artery angiography [72]. The experience of the cardiologists was not related to the patient received dose. In the proposed ANN, the duration of the surgery was not a significant contributor. Impressively, publications which investigate the role of the operative time could not be retrieved. Fluoroscopy time is mainly employed in most of the reports. For example, Chida et al. found a good correlation (r=0.801, p<0.0001) between radiation dose and fluoroscopic time for the radiofrequency catheter ablation procedures but not for the percutaneous coronary intervention procedures (r=0.628, p<0.0001) [73]. The ANNs as models, which employ a dynamic concept for interpreting outcomes and which are capable of adjusting their indigenous framework with respect to a functional target, incorporate criteria that are of importance individually, and as such could be more efficient in predicting outcomes than conventional regression models [11]. They have been used extensively in medicine. During the last years, many papers on ANNs in Neurosurgery have been also published. The networks were applied in terms of diagnosis, prognosis, outcome prediction, and biomechanical assessments [12]. Table 5 presents some indicative publications [11,13,18,19,74-78]. Interestingly, functional Neurosurgery is rarely represented [17-19]. For example, McCartney et al. collected online questionnaire responses from 607 patients with facial pain syndromes [18]. The authors designed an ANN to diagnose various facial syndromes (trigeminal neuralgia, nervus intermedius neuralgia, glossopharyngeal neuralgia, temporomandibular joint disorder, and atypical facial pain) with great accuracy. The first attempt to apply an ANN in the diagnosis of facial syndromes goes back to 2006. Limonadi et al. examined 100 patients with facial pain, who were asked to fill in a facial pain questionnaire [17]. After an interview, a formal diagnosis was assigned to each patient. The ANN was able to retrospectively predict the correct diagnosis for 95 of 100 patients (95%), and prospectively determine a correct diagnosis of trigeminal neuralgia Type 1 (sensitivity: 84%; specificity: 83%) in 43 new patients. The ability of the ANN to accurately predict a correct diagnosis for the remaining types of facial pain was limited by the sample size [17]. Furthermore, Hosen used an ANN to select the best features and to discriminate between essential and parkinsonian tremor using spectral analysis of tremor time-series recorded by accelerometry and surface EMG signal [19]. He reported an ANN-efficiency of 87.5% in both feature extraction and in pattern matching tasks in a complete classification system. Our model exhibited a sensitivity of 1, a specificity of 0.43, and an accuracy of 0.714. Even though the reported accuracy is not optimal (in the authors’ opinion, due to the small sample size), our results support the further evaluation of the ANN-methodology in future research. The current pilot study has some constraints. To start with, the sample size is small and, thus, the training and validating groups contain a limited number of patients. Further studies with a larger series of patients are warranted. Secondly, the Body-Mass-Index (BMI) could not be retrieved from the patient records and incorporated in the ANN. This is important because it has been shown that, when increasing patient thickness, radiation doses are increased [79]. Smuck et al. recorded the fluoroscopy times for 202 patients and the total procedure times for 137 patients in whom spine injections were performed [52]. The authors found a 30% increase in the mean fluoroscopy time (p=0.032), and a 35% increase in mean procedure time (p=0.031) in the group of overweight patients. In addition, Hanu-Cernat et al. reported on 127 patients treated with fluoroscopically guided spinal procedures [53]. They confirmed a correlation between weight and DAP (r=0.23, p<0.05). In other studies, the weight of the patient had no significant influence on the received dose [54]. For example, Chida et al. studied 200 cardiac intervention procedures and found a poor correlation between body weight and radiation dose (r=0.434, p<0.05) [73]. The lack of an exact diagnosis in our population is also an important drawback. Many publications underline the correlation of the diagnosis with the received radiation. A prospective study conducted on 100 patients treated with TFESIs showed that the patients with lumbar spinal stenosis had a statistically significant higher exposure time (p=0.037) as compared to patients with a herniated nucleus pulposus [46]. Hanu-Cernat et al. reported that patients with spinal pathology had higher radiation exposure than those without (r=- 0.28, p<0.005) [53]. Although the ANN-methodology presents many advantages [10,11], it exhibits some disadvantages too [12]: (a) the determination of the optimal algorithms is empirical; (b) the extraction of explicit relationships between predictors and outcomes is difficult; (c) the propensity to overfit the model during the training phase is evident.
Table 5: Indicative publications on artificial neural networks (ANNs) in neurosurgery [11,13,18,19,74-78].

Conclusion
The current study suggests that it is possible to construct with the aid of an advanced analytics tool an ANN, which can predict if the radiation burden during an ePRF procedure will be higher than the average one. Our findings have a practical relevance in the context of a better preoperative planning of ePRF interventions. The same concept could be applied in the investigation of the radiation exposure during spinal cord stimulation (SCS) surgeries or during intrathecal catheter tip placements.
References
- Byrd D, Mackey S (2008) Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep 12(1): 37-41.
- Imani F (2012) Using pulsed radiofrequency for chronic pain. Anesth Pain Med 1: 155-156.
- Cahana A, Van ZJ, Macrea L, Van KM, Sluijter M (2006) Pulsed radiofrequency: current clinical and biological literature available. Pain Med 7: 411-423.
- Shabat S, Pevsner Y, Folman Y, Gepstein R (2006) Pulsed radiofrequency in the treatment of patients with chronic neuropathic spinal pain. Minim Invasive Neurosurg 49: 147-149.
- Chua NHL, Vissers KC, Sluijter ME (2011) Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir 153: 763-771.
- Van BK, Van EM, Brinkhuize T, Patijn J, Van KM, Van ZJ (2008) Radiofrequency and pulsed radiofrequency treatment of chronic pain syndromes: the available evidence. Pain Pract 8: 385-393.
- Meisinger QC, Stahl CM, Andre MP, Kinney TB, Newton IG (2016) Radiation protection for the fluoroscopy operator and staff. Am J Roentgenol 19: 1-10.
- Sailer AM, Vergoossen L, Paulis L, Van Zwam WH, Das M, et al. (2017) Personalized feedback on staff dose in fluoroscopy-guided interventions: a new era in radiation dose monitoring. Cardiovasc Intervent Radiol 40: 1756-1762.
- Zou J, Han Y, So SS (2008) Overview of artificial neural networks. Methods Mol Biol 458: 15-23.
- Tu JV (1996) Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 49: 1225-1231.
- Matis GK, Chrysou OI, Silva D, Karanikas MA, Baltsavias G, et al. (2016) Prediction of lumbar disc herniation patients’ satisfaction with the aid of an artificial neural network. Turk Neurosurg 26: 253-259.
- Azimi P, Mohammadi HR, Benzel EC, Shahzadi S, Azhari S (2017) Use of artificial neural networks to decision making in patients with lumbar spinal canal stenosis. J Neurosurg Sci 61: 603-611.
- Wang L, Buchanan TS (2002) Prediction of joint moments using a neural network model of muscle activations from EMG signals. IEEE Trans Neural Syst Rehabil Eng 10: 30-37.
- Skoch J, Tahir R, Abruzzo T, Taylor JM, Zuccarello M, et al. (2017) Predicting symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network in a pediatric population. Childs Nerv Syst 33(12): 2153-2157.
- Jan S, Wang Y, Aghaei F, Qiu Y, Zheng B (2017) Improving performance of breast cancer risk prediction by incorporating optical density image feature analysis: an assessment. Acad Radiol S1076-6332(17): 30364-30371.
- Disse E, Ledoux S, Bétry C, Caussy C, Maitrepierre C, et al. (2017) An artificial neural network to predict resting energy expenditure in obesity. Clin Nutr 37(5): 1661-1669.
- Limonadi FM, McCartney S, Burchiel KJ (2006) Design of an artificial neural network for diagnosis of facial pain syndromes. Stereotact Funct Neurosurg 84(5): 212-220.
- McCartney S, Weltin M, Burchiel KJ (2014) Use of an artificial neural network for diagnosis of facial pain syndromes: an update. Stereotact Funct Neurosurg 92(1): 44-52.
- Hosen A (2013) A neural network approach for feature extraction and discrimination between parkinsonian tremor and essential tremor. Technol Health Care 21(4): 345-356.
- Oliveira AA, Lipinski CF, Pereira EB, Honorio KM, Oliveira PR, et al. (2017) New consensus multivariate models based on PLS and ANN studies of sigma-1 receptor antagonists. J Mol Model 23(10): 302.
- Trevathan JK, Yousefi A, Park HO, Bartoletta JJ, Ludwig KA, et al. (2017) Computational modeling of neurotransmitter release evoked by electrical stimulation: nonlinear approaches to predicting stimulation-evoked dopamine release. ACS Chem Neurosci 8(2): 394-410.
- Servomaa A, Karppinen J (2001) The dose-area product and assessment of the occupational dose in interventional radiology. Radiat Prot Dosimetry 96(1-3): 235-236.
- http://www.omar-pasha.com/index.php/en/information/pasha-electrode.html
- Setiono R, Hui LK (1995) Use of a quasi-Newton method in a feedforward neural network construction algorithm. IEEE Trans Neural Netw 6: 273-277.
- Greiner M, Pfeiffer D, Smith RD (2000) Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 45(1-2): 23-41.
- Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143(1): 29-36.
- Abejόn D, Reig E (2003) Is pulsed radiofrequency a neuromodulation technique? Neuromodulation 6(1): 1-3.
- Sluijter ME, Van Kleef M (2007) Pulsed radiofrequency. Pain Med 8(4): 388-389.
- Van Boxem K, Huntoon M, Van Zundert J, Patijn J, Van Kleef M (2014) Pulsed radiofrequency: a review of the basic science as applied to the pathophysiology of radicular pain. Reg Anesth Pain Med 39(2): 149-159.
- Bogduk N (2006) Pulsed radiofrequency. Pain Med 7(5): 396-407.
- Chao SC, Lee HT, Kao TH, Yang MY, Tsuei YS, et al. (2008) Percutaneous pulsed radiofrequency in the treatment of cervical and lumbar radicular pain. Surg Neurol 70(1): 59-65.
- Abejόn D, Garciadel Valle S, Fuentes ML, Gόmez Arnau JI, Reig E, et al. (2007) Pulsed radiofrequency in lumbar radicular pain: clinical effects in various etiological groups. Pain Pract 7(1): 21-26.
- Vigneri S, Sindaco G, Gallo G, Zanella M, Paci V, et al. (2014) Effectiveness of pulsed radiofrequency with multifunctional epidural electrode in chronic lumbosacral radicular pain with neuropathic features. Pain Physician 17(6): 477-486.
- Martin DC, Willis ML, Mullinax LA, Clarke NL, Homburger JA, et al. (2007) Pulsed radiofrequency application in the treatment of chronic pain. Pain Pract 7(1): 31-35.
- Broadman LM (2004) Radiation risk management during fluoroscopy for interventional pain medicine physicians. Curr Pain Headache Rep 8(1): 49-55.
- Shope TB (1996) Radiation-induced skin injuries from fluoroscopy. Radiographics 16(5): 1195-1199.
- Botwin KP, Fuoco GS, Torres FM, Gruber RD, Bouchlas CC, et al. (2003) Radiation exposure to the spinal interventionalist performing lumbar discography. Pain Physician 6(3): 295-300.
- Zhou Y, Singh N, Abdi S, Wu J, Crawford J, et al. (2005) Fluoroscopy radiation safety for spine interventional pain procedures in university teaching hospitals. Pain Physician 8(1): 49-53.
- Fitousi NT, Efstathopoulos EP, Delis HB, Kottou S, Kelekis AD, et al. (2006) Patient and staff dosimetry in vertebroplasty. Spine 31: E884-E889.
- Harstall R, Heini PF, Mini RL, Orler R (2005) Radiation exposure to the surgeon during fluoroscopically assisted percutaneous vertebroplasty. Spine 30(16): 1893-1898.
- Perisinakis K, Damilakis J, Theocharopoulos N, Papadokostakis G, Hadjipavlou A, et al. (2004) Patient exposure and associated radiation risks from fluoriscopically guided vertebroplasty or Kyphoplasty. Radiology 232(3): 701-707.
- Bindal RK, Glaze S, Ognoskie M, Tunner V, Malone R, et al. (2008) Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J Neurosurg 9(6): 570-573.
- Jones DP, Robertson PA, Lunt B, Jackson SA (2000) Radiation exposure during fluoroscopically assisted pedicle screw insertion in the lumbar spine. Spine 25(12): 1538-1541.
- Mroz TE, Abdullah KG, Steinmetz MP, Klineberg EO, Lieberman IH (2011) Radiation exposure to the surgeon during percutaneous pedicle screw placement. J Spinal Disord Tech 24(4): 264-267.
- Perisinakis K, Theocharopoulos N, Damilakis J, Katonis P, Papadokostakis G, et al. (2004) Estimation of patient dose and associated radiogenic risks from fluoroscopically guided pedicle screw insertion. Spine 29(14): 1555-1560.
- Botwin KP, Thomas S, Gruber RD, Torres FM, Bouchlas CC, et al. (2002) Radiation exposure of the spinal interventionalist performing fluoroscopically guided lumbar transforaminal epidural steroid injections. Arch Phys Med Rehabil 83(5): 697-701.
- Fink GE (2009) Radiation safety in fluoroscopy for neuraxial injections. AANA J 77(4): 265-269.
- Hoang JK, Yoshizumi TT, Toncheva G, Gray L, Gafton AR, et al. (2011) Radiation dose exposure for lumbar spine epidural steroid injections: a comparison of conventional fluoroscopy data and CT fluoroscopy techniques. AJR Am J Roentgenol 197(4): 778-782.
- Hwang YM, Lee MH, Kim SJ, Lee SW, Chung HW, et al. (2015) Comparison of radiation exposure during fluoroscopy-guided transforaminal epidural steroid injections at different vertebral levels. Korean J Radiol 16: 357-362.
- Kim TW, Jung JH, Jeon HJ, Yoon KB, Yoon DM (2010) Radiation exposure to physicians during interventional pain procedures. Korean J Pain 23(1): 24-27.
- Manchikanti L, Cash KA, Moss TL, Pampati V (2003) Effectiveness of protective measures in reducing risk of radiation exposure in interventional pain management: a prospective evaluation. Pain Physician 6(3): 301-305.
- Smuck M, Zheng P, Chong T, Kao MC, Geisser ME (2013) Duration of fluoroscopic-guided spine interventions and radiation exposure is increased in overweight patients. PMR 5: 291-296.
- Hanu Cernat DE, Duarte R, Raphael JH, Mutagi H, Kapur S, et al. (2012) Type of interventional pain procedure, body weight, and presence of spinal pathology are determinants of the level of radiation exposure for fluoroscopically guided pain procedures. Pain Pract 12(6): 434-439.
- Slegers AS, Gültuna I, Aukes JA, Van Gorp EJJAA, Blommers FMN, et al. (2015) Coaching reduced the radiation dose of pain physicians by half during interventional procedures. Pain Pract 15(5): 400-406.
- Manchikanti L, Cash KA, Moss TL, Pampati V (2002) Radiation exposure to the physician in interventional pain management. Pain Physician 5(4): 385-393.
- Balter S, Schueler BA, Miller DL, Wagner LK, Zelefsky MJ (2010) Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology 254(2): 326-341.
- Geleijns J, Wondergem J (2005) X-ray imaging and the skin: radiation biology, patient dosimetry and observed effects. Radiat Prot Dosimetry 114(1-3): 121-125.
- Wagner LK, Eifel PJ, Geise RA (1994) Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol 5(1): 71-84.
- Fish DE, Kim A, Ornelas C, Song S, Pangarkar S (2011) The risk of radiation exposure to the eyes oft he interventional pain physician. Radiol Res Pract 609537
- Vano E, Gonzalez L, Fernández JM, Haskal ZJ (2008) Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology 248(3): 945-953.
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, et al. (2003) Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci 100(24): 13761-13766.
- Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington (2009) Risks associated with low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology 251(1): 6-12.
- Tse V, Lising J, Khadra M, Chiam Q, Nugent R, et al. (1999) Radiation exposure during fluoroscopy: should we be protecting our thyroids? Aust N Z J Surg 69(12): 847-848.
- Zadeh HG, Briggs TW (1997) Ionising radiation: are orthopedic surgeons’ offspring at risk? Ann R Coll Surg Engl 79(3): 214-220.
- Hernandez RJ, Goodsitt MM (1996) Reduction of radiation dose in pediatric patients using pulsed fluoroscopy. AJR Am J Roentgenol 167(5): 1247-1253.
- Miller DL, Balter S, Schueler BA, Wagner LK, Strauss KJ, et al. (2010) Clinical radiation management for fluoroscopically guided interventional procedures. Radiology 257(2): 321-332.
- Chida K, Katto M, Kagaya Y, Zuguchi M, Saito H, et al. (2010) Radiation dose and radiation protection for patients and physicians during interventional procedure. J Radiat Res 51(2): 97-105.
- Oddy MJ, Aldam CH (2006) Ionising radiation exposure to orthopaedic trainees: the effect of sub-specialty training. Ann R Coll Surg Engl 88(3): 297-301.
- Bor D, Sancak T, Toklu T, Olgar T, Ener S (2008) Effects of radiologists’ skill and experience on patient doses in interventional procedures. Radiat Prot Dosimetry 129: 32-35.
- Jorgensen JE, Rubenstein JH, Goodsitt MM, Elta GH (2010) Radiation doses to ERCP patients are significantly lower with experienced endoscopists. Gastrointest Endosc 72(1): 58-65.
- Ritter M, Siegel F, Krombach P, Martinschek A, Weiss C, et al. (2013) Influence of surgeon’s experience on fluoroscopy time during endourological interventions. World J Urol 31(1): 183-187.
- Mesbahi A, Aslanabadi N, Mehnati P (2008) A study on the impact of operator experience on the patient radiation exposure in coronary angiography examinations. Radiat Prot Dosimetry 132(3): 319-323.
- Chida K, Saito H, Otani H, Kohzuki M, Takahashi S, et al. (2006) Relationship between fluoroscopic time, dose-area product, body weight, and maximum radiation skin dose in cardiac interventional procedures. AJR Am J Roentgenol 186(3): 774-778.
- Azimi P, Benzel EC, Shahzadi S, Azhari S, Mohammadi HR (2014) Use of artificial neural networks to predict surgical satisfaction in patients with lumbar spinal canal stenosis: clinical article. J Neurosurg Spine 20(3): 300-305.
- Dumont TM, Rughani AI, Tranmer BI (2011) Prediction of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network: feasibility and comparison with logistic regression models. World Neurosurg 75(1): 57-63.
- Azimi P, Mohammadi HR (2014) Predicting endoscopic third ventriculostomy success in childhood hydrocephalus: an artificial neural network analysis. J Neurosurg Pediatr 13: 426-432.
- Arle JE, Perrine K, Devinsky O, Doyle WK (1999) Neural network analysis of preoperative variables and outcome in epilepsy surgery. J Neurosurg 90(6): 998-1004.
- Amaritsakul Y, Chao CK, Lin J (2013) Multiobjective optimization design of spinal pedicle screws using neural networks and genetic algorithm: mathematical models and mechanical validation. Comput Math Methods Med 462875.
- Vano E, Gonzalez L, Fernandez JM, Prieto C, Guibelalde E (2006) Influence of patient thickness and surgery modes on occupational and patient radiation doses in interventional cardiology. Radiat Prot Dosimetry 118(3): 325-330.
© 2020 Georgios Matis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






