- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Clinical Predictors of the Evolving Ischemic Stroke According to the Tree-Structured Model
Irina Gontschar1* and Igor Prudyvus2
1Student, Health Information Management and Insurance Billing Program by the EVANS Community Adult School, USA
2Chief Application Support Analysts, EPAM Systems, Belarus, Europe
*Corresponding author: Irina Gontschar, Health Information Management and Insurance Billing Program by the EVANS Community Adult School, Los Angeles, California, USA
Submission: July 25, 2019;Published: September 18, 2019

ISSN 2637-7748
Volume2 Issue5
Abstract
Introduction: The purpose of the study is to identify the independent clinical predictors of the evolving ischemic stroke (EIS) according to the tree-structured model.
Methods and Materials: The objects of the study were 1421 patients with ischemic stroke (IS), hospitalized within 48 hours from the development of the initial symptoms. Patients with IS were admitted to the 5th Minsk City Clinical Hospital and the Minsk Emergency Hospital (Belarus) in 2002-2014 years. Evolving clinical course of the stroke is defined as an increase in the severity of neurological deficit by 2 or more points on the NIHSS scale or the death of the patient during the first seven days of hospitalization. The research is characterized due to the prospective-data-collection, and the retrospective evaluation design. The statistical method of decision trees and an algorithm of the conditional inference trees were used to create the prognostic model of EIS. Statistical data analysis was carried out applying the software packages of R V.3.2.5 and IBM SPSS Statistics 26.0.
Results: The rate of EIS reached 30%. The patients with EIS were 72.6±10.2 years old, patients without EIS-68.1±11.3 years; p = 0.005. Previously, 22 clinical, demographic, laboratory variables accommodated in the computer database were included in the conditional inference trees statistical algorithm. The prognostic statistical model of EIS has been constructed. The following independent predictors of evolving IS were identified: the stroke subtype according to the Oxford Community Stroke Project classification, the serum urea level, and red blood cell number in the total blood count. The accuracy of the statistical model reaches 0.77 (95% CI: 0.75; 0.80), the sensitivity is 0.52, the specificity-0.88, PPV-0.66, and NPV- 0.81; p < 0.001.
Conclusion: The tree-structural model allowed us to identify the independent clinical predictors of EIS.
Keywords: Cerebral infarction; Clinical characteristics; Clinical course; Conditional inference trees algorithm; Decision tree; Evolving ischemic stroke; Model; Predictor; Prognosis; Stroke deterioration
Abbreviations
AF: Atrial Fibrillation; AH: Arterial Hypertension; BMI: Body Mass Index; BUN/Cr: Blood Urea Nitrogen to Creatinine Ratio; BUN: Blood Urea Nitrogen; CART: Classification and Regression Trees; CI: Confidence Interval; CT: Computer Tomography; DBP: Diastolic Blood Pressure; ECG: Electrocardiogram; EIS: Evolving Ischemic Stroke; END: Early Neurological Deterioration; IQR: Interquartile Range; IS: Ischemic Stroke; LACS: Lacunar Syndrome; LMWH: Low-Molecular-Weight Heparins; MRI: Magnetic Resonance Imaging; mRS: Modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; No EIS: Non-Evolving Ischemic Stroke; NPV: Predictive Value of the Negative Test Result; OCSP: Oxfordshire Community Stroke Project; OR: Odds Ratio; PACS: Partial Anterior Circulation Syndrome; PBP: Pulse Blood Pressure; POCS: Posterior Circulation Syndrome; PPV: Predictive Value of the Positive Test Result; Q1: Lower Quartile; Q3: Upper Quartile; RBC: Red Blood Cells; RSPCNN: The Republican Scientific and Practical Center for Neurology and Neurosurgery of the Ministry of Health of the Republic of Belarus; rtPA: Recombinant Tissue Plasminogen Activator; SBP: Systolic Blood Pressure; SD: Standard Deviation; SSS: Scandinavian Stroke Scale; TACS: Total Anterior Circulation Syndrome; TIA: Transient Ischemic Attack; TOAST: Trial of ORG 10172 in Acute Stroke Treatment; UFN: Unfractionated Heparin; WBC: White Blood Cells; WHO: World Health Organization
Introduction
The Republic of Belarus is the upper-middle-income country in Eastern Europe, with 9.46 million of the citizens [1]. For the Belarusian population, life expectancy at birth has been arranged up to 74 years [1]. At the same time, the lifetime risk of the ischemic stroke (IS) developing reaches 21% [2]. According to the Global Burden of Disease Study [3], in 2017 the incidence of the brain infarct in Belarus exceeded thirty thousand cases per year, the value of deaths caused by IS-more than ten thousand. Every third patient with IS, hospitalized in a specialized stroke department of the clinic, has an elevation of the stroke symptoms severity [4-8]. The previously published studies showed a close relationship of the evolving clinical course of stroke with an unfavorable outcome and increased lethality [9-13]. Earlier [14], we have determined that the 5-year survival rate of patients with non-evolving IS reaches 0.57; 95% confidence interval (CI): 0.54-0.61. In the case of the evolving ischemic stroke (EIS), the survival rate during the same observation time drops to 0.30; 95% CI: 0.26-0.36.
During 2002-2015, based on data from research conducted on the different aspects of IS problem, the authors of the article created a computerized dataset of patients hospitalized in the stroke departments of the 5th Minsk City Clinical Hospital and the Minsk Emergency Hospital. Both hospitals are clinical bases of the Republican Scientific and Practical Center for Neurology and Neurosurgery of the Ministry of Health of the Republic of Belarus (RSPCNN). The researcher associates of RSPCNN are also neurologists-consultants of the stroke departments of hospitals, providing inpatient care to people of Minsk city. Such cooperation enables investigators not only to directly participate in diagnostic, therapeutic and prophylactic measures conducted in the clinic but also to carry out a scientifically based analysis of the clinical data of patients of the corresponding stroke departments. The purpose of the study is to identify the independent clinical predictors of EIS by creating a tree-structured model.
Methods and Materials
Study design
The objects of the study were 1421 randomly selected patients with IS, admitted within 48 hours from the development of the initial symptoms. The diagnosis of IS was based on the World Health Organization (WHO) criteria [15], confirming it in 100% observations by neuroimaging investigation - computer (CT) and/ or magnetic resonance imaging (MRI) of the brain. The severity of neurological disorders was evaluated using the National Institutes of Health Stroke Scale (NIHSS) [16]. The functional outcome of IS was measured at the discharge from the clinic by the modified Rankin Scale (mRS) [17].
The end point of the study is the development of EIS. Evolving clinical course of the stroke is defined as an increase of the neurological deficit gravity by 2 or more points on the NIHSS scale or the death of the patient during the first seven days of stay in the clinic. The underlying reasons of EIS were the brain infarction hemorrhagic transformation, brain edema, recurrent ischemic stroke, pneumonia, urinary infection, cardiovascular and gastrointestinal complications, seizure [8]. The research is characterized due to the prospective-data-collection, and the retrospective evaluation design. The main criterion for inclusion in the observed cohort of patients was the presence of acute IS, which developed up to 48 hours before hospitalization, in adults. The intracranial hemorrhage, venous sinus thrombosis, transient ischemic attack (TIA), traumatic brain injury, likewise oncological, autoimmune, or degenerative diseases of the central nervous system were exclusion criteria from research.
The ethical committee of RSPCNN approved the study protocol. Informed consent was gotten from all patients or their representatives. The research was conducted according to the Good Clinical Practice principles and the Declaration of Helsinki [18]. Data were collected during surveys conducted by neurologists. Information about the stroke premorbid period was obtained from the patients, their representatives, as well as from the submitted medical documentation. The survey was performed in Russian. Information on the arterial hypertension (AH), coronary heart disease, atrial fibrillation (AF) and other cardiac arrhythmias, angina pectoris, myocardial infarction, diabetes mellitus, peripheral arteries and veins disorders, pathology of the respiratory organs, gastrointestinal tract, kidneys and urinary tract, brain traumatic injuries, previous TIA, ischemic and hemorrhagic strokes, height, weight, body mass index (BMI) was input into the registration paper form of the patient with IS. The presence of cardiovascular risk factors was determined using physical examination, interview, laboratory, and instrumental methods of investigation. The received information was entered into the specially developed computer dataset, which included demographic, clinical, administrative data, information about drug therapy, and the modification of neurological symptoms using specialized stroke scales. The research team also has analyzed data on the evolution of neurological deficit from the start of the initial symptoms to the patient’s delivery to the emergency department.
In addition to CT or MRI, all patients underwent a 12-channel electrocardiogram (ECG), an x-ray of the chest organs, and laboratory tests. If possible, the in-patients were examined with an ultrasound examination of the extracranial and intracranial arteries, echocardiography, and Holter ECG study. Complete blood count, chemical profile, lipid profile, hemostasiogram were performed in clinical laboratories of the 5th Minsk City Clinical Hospital and the Minsk Emergency Hospital.
The research team has brought into the database information about systolic blood pressure (SBP), and diastolic blood pressure (DBP) on the onset of stroke, and also in the emergency department. Additionally, we calculated the value of pulse blood pressure (PBP) - the difference between SBP and DBP in patients with IS. When structuring the information received, the localization of IS was determined by the cerebral vascular territories. The stroke subtypes were defined according to the Oxfordshire Community Stroke Project (OCSP) criteria [19] and the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification [20]. A detailed description of the study design was published earlier [7,8,14,21].
Information on the in-patient therapy was subject to particular registration. The prescription of antiplatelet, anticoagulant, antihypertensive, antiarrhythmic drugs, diuretics, cardiac glycosides, and the aldosterone receptor antagonist was taken into account. The observed cohort did not conclude individuals who received thrombolytic therapy. On the whole, the treatment of patients with IS in the stroke department was carried out in accordance with the Clinical protocols for the diagnosis and treatment of patients with pathology of the nervous system, approved by the Ministry of Health of the Republic of Belarus in 2005 [22].
Statistical analysis
The computerized dataset was organized and analyzed using standard statistical methods. The relevant results were carried out applying the software packages of R V.3.2.5 [23,24] and IBM SPSS Statistics 26.0.
The research team has validated the Gaussian distribution of the continuous variables using Shapiro-Wilk’s test, and the normal quantile-quantile plots [25-28]. Quantitative parameters with the normal distribution were presented as a mean and a standard deviation (SD). Otherwise, the quantitative variables were shown as a median and interquartile range (IQR), that is, the first quartile and the third quartile (Q1-Q3) [29,30]. Quantitative data with Gaussian distribution were compared using a Student test (for two groups) or ANOVA (for three or more groups). Post hoc Tukey test was engaged in estimating the differences between three or more groups. If the data distribution was not normal, the Wilcoxon- Mann-Whitney test was employed to compare two groups. The Kruskal-Wallis test was used for three or more groups. After that, post hoc analysis was accomplished on the basis of the Kruskal test for multiple comparisons and the BDM test [31]. Information about the qualitative parameters and grouped quantitative variables distribution were provided as frequency distributions indicating the parameter category (if the number of observations was reasonably high) and/or as an absolute number of cases [32,33]. For comparison of the qualitative variables that can be depicted in the form of contingency tables 2×2 was given the two-tailed Fisher exact test [34]. If the qualitative stratified data can be rated as k contingency tables of 2×2, the Cochran-Mantel-Haenszel test was all pied with the Monte Carlo evaluation of p [35]. We used the odds ratio (OR) for the quantitative description of the relationship of the distinct variable to the end point of the study. OR was computed as one of the results of the statistical assessment by comparing frequencies in different groups. At the previous stages of the investigation, we performed an univariate statistical analysis of the clinical, demographic, laboratory, and administrative characteristics of patients with EIS and without EIS [7,8,13,14,21,36]. Statistics are computed using non-missing data only.
The statistical method of the decision trees was used to create the prognostic model of EIS. This method of statistical analysis is gaining increasing popularity in published studies that is explained by the possibilities of automated identification of logical data patterns [37]. The discovered data patterns are graphically displayed as a prognostic model of decision tree-dendrogram [32]. Dendrogram demonstrates the significance of the effect of the variables included in the model on the outcome. We applied the conditional inference tree calculation algorithm for constructing the decision tree [38-41]. As the end point of the study (that is, as a dichotomous outcome of the model), we determined the presence or absence of EIS. In this way, a predictive model based on decision trees made it possible to solve the problem of classifying the variants of the clinical stroke course. Under the positive class, when performing the classification, we understood the presence of the evolving clinical course of the cerebral infarction.
Creating the dendrogram consists of four steps:
a. Defining the attribute criteria used to branch the decision tree;
b. Establishing the conditions for further data splitting (node) or stopping the growth of the tree (leaf or terminal node);
c. Determination of cut-off rules for obtaining the model of a practically meaningful decision tree, fitting and inspecting the tree;
d. Evaluation of the accuracy of data classification - calculation of the characteristics of the resulting model.
According to Hothorn et al. [38,39], in the conditional inference tree algorithm, the key for the creating of the interpretable decision tree is the detachment of variable picking and binary splitting technique into the first and second steps. The construction of the dendrogram takes place from top to bottom - descending. When creating a binary decision tree, each node in the tree forms two daughter branches. Daughter branches, in turn, can also be divided into two parts several times. The growth of each branch ends with the formation of terminal nodes or leaves [37,42-44]. In our model, the five terminal nodes represent information on the distribution of patients with progressive and regressive stroke in the presence of the composition of the specific clinical characteristics. Decision trees make it possible to present the classifying rules for parameters in the hierarchical, consistent structure. Each step of the tree construction proceeds according to the rule formed in the examination node, that is, according to the branching conditions of the dendrogram. The tree embranchment rule is understood as the logical construction, offered in the form “if ... then ...” [32]. In each node of the dendrogram, the given parameters’ set is divided into two parts. One part of the characteristics represents the proportion of the analyzed parameters for which the classification rule is satisfied. This circumstance allows us to refer the patient to the EIS group. For another part of the data, the classification condition is not met. In this case, the patient with similar features belongs to the group of the non-evolving clinical course of IS. The virtue of each split is examined by two-sample linear statistics [38,39].
Decision trees analysis refers to the probabilistic-logical methods for the formation of the crucial rule based on the algorithm for composition the binary classification tree. The undoubted advantages of the decision trees include the transparency of the decision-making mechanism [44]. The simplicity and visibility of the results graphical interpretation is obvious even to the untrained user on an intuitive level [45]. The influence of the factors included in the model was considered significant at p<0.05. Further, we clarified the accuracy of our tree model using composite methods. Random forests and boosting statistical approaches were implemented to refine the predictions proceed from the decision tree [37,46]. In improving the predictive model, we used the classification type of random forest. Five hundred copies of the tree were grown. The out-of-bag (OOB) estimates of error rate constitute 23.89%. As the information measure for selecting the current covariate, the Gini index was also computed for the variables included in the decision tree construction algorithm. The variables of interest screening were implemented for the following multivariate analysis. For this, the value p=0.1 has been taking as the threshold level. The impact of the variable was considered marginal if the probability of the null hypothesis was in the range of 0.05-0.10. Such characteristics were enrolled in the tree-structured model for the following estimate. The conclusion to admit or reject the null hypothesis (2-sided tests) was pulled off applying p=0.05 as the threshold value. Differences were regarded as statistically significant at p-value<0.05.
Results
Characteristics of the patients with IS
Table 1 demonstrates the clinical, demographic, administrative, therapeutic, and laboratory characteristics by IS cohort. Of the 1421 cases of IS, the mean patient age reached 69.5±11.1 years. In this cohort of patients, 19 (1.3%) were in the age group of 25- 44 years, 274 (19.3%)-45-59 years, 598 (42.1%)-60-74 years, and more than one third of the entire cohort of patients-530 (37.3%), made up people from 75 years and older. Of these patients, 655 (46.8%) were male, and 756 (53.2%) - female. The race of all patients was designated as white. According to the clinical examination and neuroimaging (CT, MRI), the topography of brain infarction lesions was determined. The most substantial fraction in the stroke localization structure consisted of IS in the left anterior circulation-594 (41.8%) patients, followed by IS in the right anterior circulation-435 (30.6%), posterior cerebral circulation-352 (24.8%), and multifocal stroke territory-40 (2.8%) cases. On the first days of hospitalization, the clinical subtype of IS was diagnosed after the neurological examination of the clinical symptoms following OCSP criteria. In the top place of incidence was partial anterior circulation syndrome (PACS)-546 (38.4%) among all 1421 patients. It was followed by lacunar stroke syndrome (LACS)-333 (23.5%) people, total anterior circulation syndrome (TACS)-320 (22.5%), and posterior circulation syndrome (POCS)- 222 (15.6%).
Table 1:Baseline characteristics of patients with ischemic stroke (n=1421). Data expressed as: n (%), mean ± SD, median (Q1-Q3).
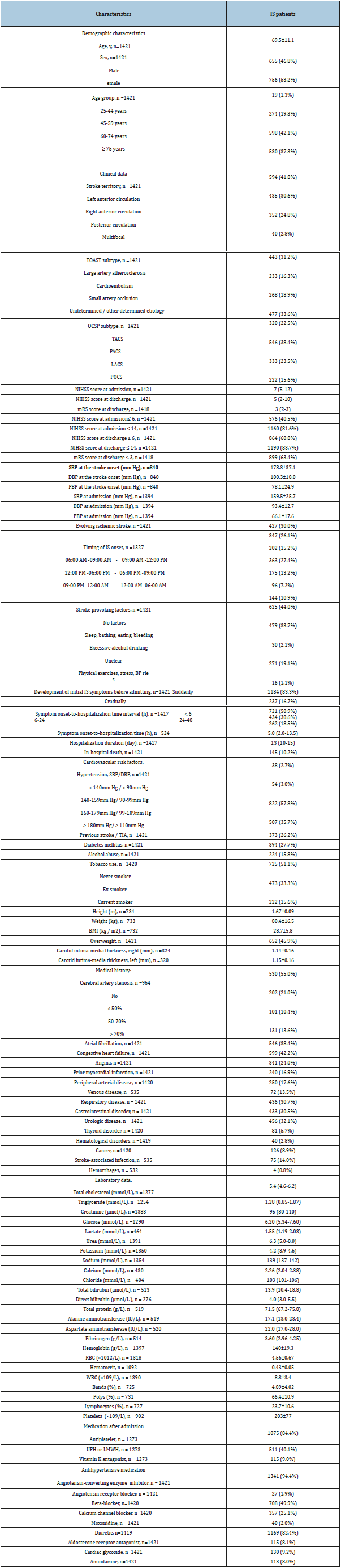
[BMI: body mass index; DBP: diastolic blood pressure; EIS: evolving ischemic stroke;IS: ischemic stroke; LACS: lacunar syndrome; LMWH: low-molecular-weight heparins; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; No EIS: non-evolving ischemic stroke; OCSP: Oxfordshire Community Stroke Project; PACS: partial anterior circulation syndrome; PBP: pulse blood pressure; POCS: posterior circulation syndrome; Q1: lower quartile; Q3: upper quartile; RBC: red blood cells; SBP: systolic blood pressure; SD: standard deviation; TACS: total anterior circulation syndrome; TIA: transient ischemic attack; TOAST: Trial of ORG 10172 in Acute Stroke Treatment; UFN: unfractionated heparin; WBC: white blood cells]
The variant of cerebral infarction, according to the TOAST criteria, was established collectively by the scientific consultant of the neurological department (IG), the attending neurologist, and the head of the neurological department. The conclusion of the neurological and somatic examination, previous medical history, the findings of electrocardiography, echocardiography, ultrasound scan of cerebral vessels, Holter heart rate monitoring, and other instrumental investigations were taken into account. TOAST stroke variants were distributed as follows: large artery atherosclerosis-443 (31.2%), cardioembolism - 233 (16.3%), small artery occlusion-268 (18.9%), and undetermined/other determined cerebral infarct etiology-477 (33.6%).
NIHSS score at admission and discharge was checked by 100% IS patients. The corresponding scale scores were 7 (5-12) and 5 (2-10) points, respectively. At the hospital admission, the mild neurological deficit not exceeding 6 NIHSS points was recorded in 576 (40.5%) patients. Initially, mild or moderate severity of IS symptoms (≤14 NIHSS score) was observed in 1160 (81.6%) of the individuals. Further, NIHSS assessment at discharge≤6 points occurred in 864 (60.8%) of the 1421 patients included in the database, and NIHSS ≤14 points-in 1190 (83.7%) persons. The estimation of functional deficiency at the end of treatment in the acute stroke department matched to 3 (2-3)mRS points (Figure 1). The analysis of the distribution of IS onset on the times of day (n=1327) showed the following results. In 347 (26.1%) patients, the first stroke signs appeared in the time interval from 6.00am to 9.00am, in 202 (15.2%)- from 9.00am to 12.00pm, in 363 (27.4%)- from 12.00pm to 6.00pm, in 175 (13.2%)-from 6.00pm to 9.00pm, in 96(7.2%)-from 9.00pm to 12.00am, and in 144 (10.9%) of persons - at night, from 12.00 am to 6.00am.
Figure 1:Tree-structured model of the evolving ischemic stroke according to the conditional inference’s trees algorithm. The accuracy model is 0.77;95%CI:0.75-0.80, sensitivity-052, specificity-0.88, PPV-0.66, and NPV- 0.81; p<0.001.
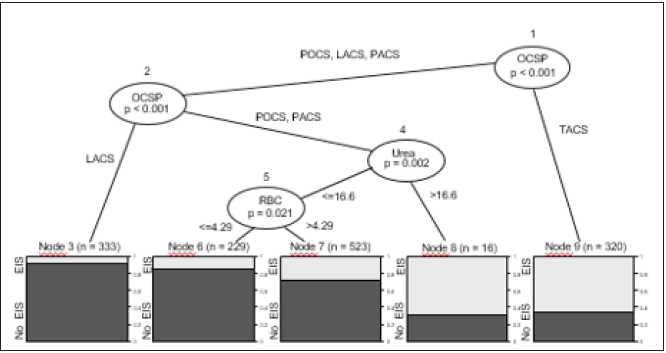
EIS: Evolving Ischemic Stroke; LACS: Lacunar Syndrome; OCSP: Oxford Shire Community Stroke Project; No EIS: Non-Evolving Ischemic Stroke; PACS: Partial Anterior Circulation Syndrome; POCS: Posterior Circulation Syndrome; RBC: Red Blood Cell Number In The Total Blood Count(×1012/l); TACS: Total Anterior Circulation Syndrome; Urea: The Serum Urea Level(mmol/L).
The majority of the interviewed patients and their family members - 625 (44.0%) cases, indicated the absence of any apparent situations and events that could trigger the stroke. Among the identified factors led to the occurrence of an acute disturbance of cerebral circulation, blood-pressure-lowering events occurred more often than others. These are stroke-provoking factors, such as sleep, bathing, heavy meal, loss of the blood volume due to bleeding-479 (33.7%) cases. Circumstances such as intense physical exercises, stress, and expressed rise in blood pressure, were indicated by 271 (19.1%) respondents. Interestingly, 30 (2.1%) patients did not conceal the fact of abundant alcohol consumption on the eve of the stroke. In 16 (1.1%) cases, the presence or absence of some IS provoking factors could not be established.
The more significant part of the patients-1184 (83.3%), were characterized by an acute IS emergence at the pre-hospital stage. In 237 (16.7%) observations, the initial stroke symptoms increased gradual, fluctuating, or steadily. The median time from the onset of stroke to the delivery of the patient (n=524) to the emergency department of the clinic was 5.0 (2.0-13.5) hours. In 1417 patients, the period from stroke to hospitalization was structured into the following time intervals: up to 6 hours, from 6 to 24 hours, and 24 to 48 hours. More than half of the patients observed-721 (50.9%) were delivered in the hospital in the first 6 hours. Another 434 (30.6%) patients were hospitalized in the time frame of 6-24 hours, and residuary 262 (18.5%) persons-on the second day from IS beginning. The median length of stay of patients with IS (n=417) in the acute stroke department of the clinics was 13 (10-15) days. Every tenth patient died during the hospital stay-145 (10.2%) of 1421 people. Cardiovascular risk factors of the study cohort are presented in Table 1. In 1421 patients with IS, 373 (26.2%) had previous stroke or TIA, 394 (27.7%) - diabetes mellitus, and 652 (45.9%) - overweight. Chronic alcohol abuse was confirmed by 224 (15.8%) interviewed individuals. Half of the stroke patients (725 (51.1%) out of 1420 people) never smoked. Another 473 (33.3%) persons were characterized as “former smokers”, and 222 (15.6%)-as “current smokers”. The presence and degree of AH were diagnosed according to ESC/ESH criteria [47] and the National Guidelines for Arterial Hypertension of the Belorussian Scientific Society of Cardiologists 48]. The normal blood pressure range with SBP up to 140mm Hg and DBP up to 90mm Hg was identified only in 38 (2.7%) of 1421 patients in the pre-stroke period. Hypertension of the first degree (SBP / DBP: 140-159 / 90-99mm Hg) occurred in 54 (3.8%) of the examined individuals, the second degree (SBP / DBP: 160-179 / 99-109mm Hg)- in 822 (57.8%), and the third degree (SBP / DBP: ≥ 180 / ≥ 110mm Hg) - in 507 (35.7%).
Ultrasound scanning of the extracranial and intracranial arteries was performed during hospitalization in the stroke department in 934 (65.7%) patients. The absence of significant atherosclerotic vessel stenosis was diagnosed in 530 (55.0%) of 934 individuals. Stenosis up to 50% of the arterial lumen was detected in 202 (21.0%) cases, stenosis of 50-70%-in 101 (10.4%), and stenosis>70% or cerebral artery occlusion -in 131 (13.6%) (Table 1). AF was found in 546 (38.4%) of 1421 patients, congestive heart failure-in 599 (42.2%), angina-in 341 (24.0%), prior myocardial infarctionin 240 (16.9%), and peripheral arterial disease-in 250 (17.6%). The frequency of respiratory disease presence accomplished 436 (30.7%) cases, gastrointestinal disorders-433 (30.5%), urologic diseases-456 (32.1%), thyroid disorders-81 (5.7%), hematological disorders-40 (2.8%), and previous or current cancer-126 (8.9%). In patients hospitalized in 2011-2014, information on peripheral veins pathology, stroke-associated infections, and hemorrhagic complications was additionally ascertained and entered into the computer dataset. The venous diseases were observed in 72 (13.5%) out of 535 patients, the infectious complications of IS-in 75 (14.0%) out of 535, the bleedings-in 4 (0.8%) out of 532.
Table 1 shows the median values of the main parameters of the blood chemical profile, including electrolytes, glucose, urea, creatinine, total protein, lipid metabolism indicators, and fibrinogen. The research team also recorded in paper and electronic forms information about the indicators of the complete blood count, such as hemoglobin, hematocrit, red blood cell (RBC) count, white blood cell (WBC), and platelet, percentage of leucocytes number such as bands, polys, and lymphocytes. Intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) and mechanical extraction of cerebral emboli was not performed in the patients participating in the study. Antithrombotic drugs were prescribed to patients with stroke immediately after hospitalization in the department for the prevention of recurrent ischemic cerebrovascular events, and for the prophylaxis of venous thromboembolism. Antiplatelet agents (acetylsalicylic acid, rarer clopidogrel) were prescribed 1075 (84.4%) of 1273 analyzed patients, unfractionated heparin (UFN) or low-molecular-weight heparins (LMWH)- in 511 (40.1%), vitamin K antagonist warfarinin 115 (9.0%). Information about the frequency of antihypertensive, diuretic, antiarrhythmic drug usage was available almost in 100% of patients with IS. The antihypertensive treatment included the following drugs: angiotensin-converting enzyme inhibitors - 1341 (94.4%) cases, angiotensin receptor blockers-27 (1.9%), betablockers- 708 (49.9%), and calcium channel blockers-357 (25.1%). Diuretics was received by 1169 (82.4%) patients, aldosterone receptor antagonist spironolactone-115 (8.1%), cardiac glycosides-130 (9.2%), and amiodarone-113 (8.0%).
Tree-structured model of EIS
Figure 1 shows the tree-structured model of EIS. The prognostic model is originated in on the clinical characteristics of 1421 stroke patients. The principal constituent of the mathematical calculations of this decision tree is the algorithm “conditional inference tree.” To construct the dendrogram, we used 21 clinical variables that demonstrated the relationship with the evolving clinical course of IS in univariate data analysis. The following clinical parameters were involved in the starting assessment: OCSP, age, gender, NIHSS score at admission, AF, previous myocardial infarction, congestive heart failure, hypertension, diabetes mellitus, alcohol abuse, previous stroke or TIA, peripheral arterial disease, SBP and DBP at admission, creatinine, glucose, urea, potassium, sodium, hemoglobin, RBC, and WBC. The decision tree starts to grow from the root node 1, which is the clinical subtype of IS according to the OCSP classification (Node 1: OCSP). If in the node 1 implements the condition “the presence of POCS, LACS, PACS” (POCS, LACS, PACS, respectively), then the dendrogram branches to the right, to the corresponding daughter node - inner node 2 (Node 2: OCSP). In the inner node 2, the fulfillment of the clause “the presence of the lacunar stroke” (LACS) leads to the terminal node 3 (Node 3).
As can be seen in Figure 1, each terminal node reflects the ratio of the evolving and non-evolving clinical course of IS. The terminal node 3 demonstrates the proportions of the EIS (light hatching) and No EIS (dark hatching) strokes for patients with lacunar IS. The classification condition “the presence of lacunar stroke” in the inner node 2 is not met when the patient has IS lesions location in the brain posterior circulation. In this case, the decision tree branches to the left, to inner node 4 (Node 4: Urea). The presence of the clause “blood urea level ≤ 16.6 mmol / L” in node 4 leads to the inner node 5 (Node 5: RBC). In the node 5, the level of RBC in the general blood count ≤ 4.29 ×1012 / L provides the decision tree grows to the right, to the terminal node 6. Node 6 performs the ratio of individuals with EIS and without EIS by the attendance of one of the two IS subtypes (PACS or POCS), as well as by the urea content in the blood plasma ≤ 16.6 mmol / L, and RBC ≤ ×1012 / L. If the number of the erythrocytes exceeds 4.29 ×1012 / L in the inner node 5, then in this point the dendrogram bifurcates to the left, to the terminal node 7. The high level of the blood urea, more than 16.6mmol/L, causes split of the dendrogram in the inner node 4 to the left, to the terminal node 8. The terminal node 8 introduces the ratio of patients with EIS and without EIS, satisfying two conditions: the presence of PACS or PACS, as well as the urea level > 16.6 mmol/L. Thereafter, being there TACS at the root node 1 leads directly to the terminal node 9.
The research team has employed stroke patients’ data from the computerized dataset and fit the conditional inference tree with 5 terminal nodes according to the statistical algorithm in Figure 2. Indeed, initially, we applied 21 different variables to the model, reflecting the clinical and demographic characteristics of patients with stroke, as well as the levels of systolic and diastolic blood pressure and indicators of the chemical profile and the general blood test. As a result, as independent prognostic factors, that are significantly associated with the evolving clinical course of IS, three clinical parameters were distinguished: OCSP stroke subtype, urea level, and RBC count (p<0.001). The first prognostic predictor, OCSP stroke subtype, in the root node 1 (Node 1: OCSP), provides the division of the decision tree into the left and right parts based on the presence or absence of TACS. In the inner node 2, the same predictor - OCSP indicates the dendrogram splitting into the left side (LACS) and the right side (POCS or PACS). The second independent predictor of the stroke course is the urea content in the blood. Moreover, the third predictor is the RBC number in the blood count test. To estimate the developed model goodness for predicting the evolving clinical course of IS, the research team computed the accuracy, sensitivity, specificity, predictive value of the results obtained using the conditional inference trees mathematical algorithm. The class predictions of the decision tree for the training sample (and for new observations as well) is counted up using the predict function. A resemblance with the true EIS class memberships is carried out by data from Table 2. The accuracy of the created statistical model is 0.77, i.e., the conditional inference trees algorithm allows us to correctly classify patients with EIS and without EIS in 77% of cases. The 95% CI of the prediction accuracy ranges from 0.75 to 0.80 (95% CI: 0.75-0.80), the sensitivity is 0.52, the specificity-0.88. The predictive value of the positive test result (PPV), equal to the probability of developing the evolving ischemic stroke variant with the positive test result, is 0.66. The predictive value of the negative study result (NPV), alike to the possibility of the EIS absence (i.e., equal to the probability of the existence of the non-evolving stroke) with the negative test result reaches 0.81.
Table 2:Conditional inference tree class probabilities for EIS.
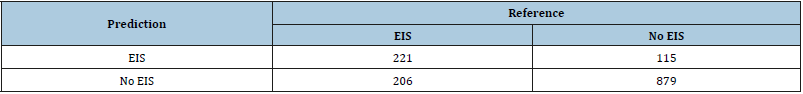
[EIS: evolving ischemic stroke; No EIS: non-evolving ischemic stroke]
Figure 2:Conditional inference tree creating algorithm.
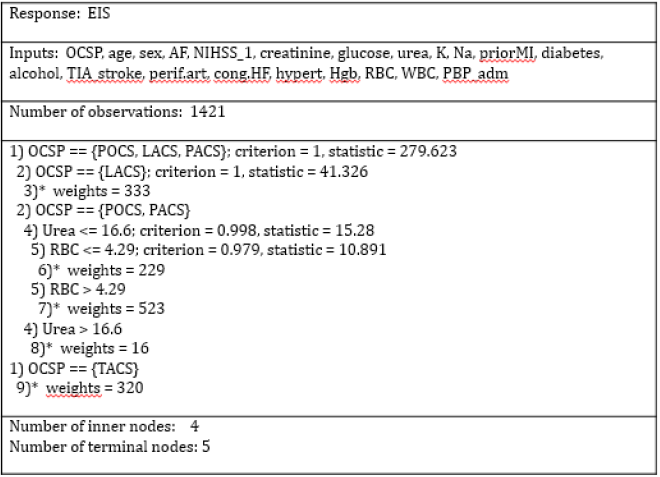
Alcohol: Alcohol Abuse; AF: Atrial Fibrillation; Cong. HF: Congestive Heart Failure; Diabetes: Diabetes Mellitus; EIS: Evolving Ischemic Stroke; Hgb: Hemoglobin; Hypert: Arterial Hypertension; K-Potassium Level In The Blood; Na-Sodium Level In The Blood; LACS: Lacunar Syndrome; NIHSS_1: National Institutes Of Health Stroke Scale At Admission; OCSP: Oxford Shire Community Stroke Project; PACS: Partial Anterior Circulation Syndrome; PBP_adm: Pulse Blood Pressure At Admission; Perif. Art: Peripheral Arterial Disease; POCS: Posterior Circulation Syndrome; priorMI: Prior Myocardial Infarction; RBC: Red Blood Cells; TACS: Total Anterior Circulation Syndrome; TIA_stroke: Previous Transient Ischemic Attack or Stroke; WBC: White Blood Cells.
The construction of dendrogram based on the methods of multivariate statistical analysis demonstrates the structure of the employed data and identifies independent prognostic criteria for the evolving clinical course of the cerebral infarction. Decision trees allow determining the belonging of patients to the groups with EIS and without EIS depending on the values of clinical and paraclinical indicators described the patient condition. The decision treestructured model graphically represents the classification process with the ranking of data by the degree of significance for the specified outcome-evolving clinical course of IS (positive class) or non-evolving stroke (negative class). So, the ischemic stroke subtype according to OCSP criteria (OCSP), the urea concentration in the blood serum (Urea), and the erythrocyte count in the general blood test (RBC) have been identified as independent prognostic factors in the decision tree analysis by conditional inference tree algorithm.
Discussion
The underlying pathophysiological mechanisms of the neurological deterioration by IS are not entirely understood [48-53]. Over six decades of research reveal the role of several pathways for increasing damage to the brain due to acute ischemic stroke [54,55]. These are factors such as ischemic injury expansion, cerebral edema, engaged artery re-occlusion, collateral circulation insufficiency, repeated IS, hemorrhagic transformation of the brain infarction [53,56-59]. It should be noted that the same patient may have one of the above pathophysiological mechanisms for the development of EIS, or the combination of them may be present [60]. In publications on the stroke-in-evolution, we can see the dividing up an early neurological deterioration (END) and the late neurological worsening [4,53,61,62]. Causes of the END in the first 2-3 days are considered to be cerebral edema, the stroke-causing blood clot enlargement, and arterial re-occlusion [52,61-66]. The evolving clinical course of IS in the next 4-7 days is usually explained by the systemic disorders of homeostasis, coagulation, strokeassociated infection, venous thromboembolism, decompensating of chronic somatic diseases, and epileptic seizures [7,8,49,56,67]. When the patient with IS receives reperfusion therapy, the study design traditionally evaluates the presence or absence of END by 4 points on the NIHSS scale for 24-72 hours [68-71]. The reasons for END of patients exposed to advanced methods of treating cerebral infarction (intravenous thrombolysis, intra-arterial thrombolysis, thrombectomy) differ from traditional ones [71-73]. Most often, the condition of patients worsens due to complications of advances in treatments, such as symptomatic hemorrhagic transformation of the infarcted lesion, up to the development of extensive intracerebral hematomas, malignant brain edema, re-occlusion of the concerned cerebral artery, non-restoration of blood circulation in the area of cerebral infarction, IS grow, extracranial bleeding [61,67,68,70,74-76]. Since thrombolysis was a contraindication for inclusion in our study, we would not want to dwell on the reasons for the progression of stroke against the background of the indicated methods of intensive restoration of cerebral circulation.
Depending on the selected rating scale, the time of EIS establishment, the features of the observed cohort, the methods of treatment used, the frequency of stroke-in-evolution ranges from 10 to 40%, averaging 30% [6,7,8,21,51,77]. The main pathophysiological mechanisms, predictors, clinical impact, and incidence of EIS were investigated using data from such impressive stroke registers as the Harvard Cooperative Stroke Registry [78,79], the Lausanne Stroke Registry [10], the European Cooperative Acute Stroke Study [4], the Besançon Stroke Registry [12], the German Stroke Study Collaboration [66], and the SITS International Stroke Thrombolysis Register [80]. In previous studies, the frequency and severity of neurological worsening were analyzed as in the four clinical categories of an acute ischemic cerebrovascular accident following the OCSP [12,81-83]. The subject of scientific research was also the evolving clinical course of pathogenetic variants of IS by the TOAST criteria [20,66,84]. The neuroimaging characteristics of EIS were examined separately: the brain tissue density decrease of 1/3 of the middle cerebral artery territory, hyperdense middle cerebral artery sign, brain edema, hemorrhagic transformation of the cerebral infarct on CT and MRI scans [50,58,63,65,85]. A reduction in cerebral blood flow and volume (rCBV) ratio (ipsilateral value/contralateral value) on perfusion MRI was an independent predictor of neurological deterioration in the first 72 hours in patients with IS in the study by Kim et al. [64].
The reasons for neurological symptoms aggravation in IS where the systemic disorders of homeostasis, which are represented in the biomarkers such as glucose, urea, C-reactive protein, fibrinogen, D-dimers, homocysteine, glycine, interleukin-6, cystatin C in the blood, WBC count as well as the content of glutamate, glycine, interleukin-6, L-arginine in cerebrospinal fluid [60,67,71,81,86-94]. The first publications describing evolving ischemic brain damage during occlusion of the basilar artery belong to Millikan [54,55]. These days, this issue is dedicated as the separate original neurological studies [8,70,77,89,95], as well as publications described the analysis of the large stroke databases [4,10,66,72,78,79].Until now, the lack of the standard definition of evolving IS has remained a very significant methodological problem. Various terms have been proposed for its definition: “progressive stroke”, “infarct progression”, “stroke with progression”, “stroke-inevolution”, “stroke with deterioration”, “neurological deterioration”, “early neurological deterioration”, “neurological worsening” [11,13,66,89,96-101].
Common to all of the above EIS names is the following: the diagnosis of evolving stroke is established when neurological deficit increase and the level of consciousness decrease after admission to the hospital are recorded in medical documents by the physicians or nurses who have special training in assessing the patient’s condition using specialized stroke scales. In the European Acute Stroke Study (ECASS), early IS worsening was defined as the consciousness decline or motor impairments of ≥2 points of the Scandinavian Neurological Stroke Scale (SSS) or the speech decrease of ≥3 SSS points during the first 24 hours of stay in the stroke department [4]. The stroke progression was outlined in the European Progressing Stroke Study (EPSS) designation as the death of the patient or any significant neurological deterioration from the stroke onset to 72 hours. Significant neurological impairment was understood according to SSS as a decrease in the motor function of the limbs, eye movement, level of consciousness, and/or a three or more point speech worsening [72,102]. In an article by Barber et al. [103], based on an analysis of data from the consecutive series of 873 patients, the IS deterioration criterion was the increase in neurological deficit by one point on SSS during the first three days of treatment [103].
It should be noted that the band of the rating stroke scales used to state the progressive clinical course, varies significantly depending on methodological approaches. So, Tei et al. [82] used the decrease ≥1 point of the Canadian Neurological Scale score to diagnose the progression of TACS, PACS, and LACS. In the same study, the authors 82] applied the total mRS score dropping of one or more points to recognize deterioration in patients with POCS within seven days follow-up. Kwan et al. [81] proposed the increase in the NIHSS score of two or more points or the lethal outcome between the first and fifth days of hospital stay as END criterion. A range of contemporary researchers suggests the neurological symptoms impairment by 2 or more NIHSS points under the term “END” during the first ten days of the brain infarct [49,104]. As a result of the research projects on the problem of stroke, the authors of this article put forward in the healthcare of the Republic of Belarus the determination of progressive ischemic stroke [7,8,36]. EIS was defined as deterioration in the level of consciousness, the local neurological deficit of 2 or more points on the NIHSS score, or the death of the patient within the first 7 days after admitting [13,21,77]. The same criteria for EIS diagnosing were selected by Geng et al. [6], Lee et al. [67], Kim et al. [92], Lin et al. [93], Zhang et al. [100], and Kanamaru et al. [101]. All the same, whatever the term, EIS is corresponding to the imperfect prognosis for ischemic stroke patients [6,49,59,61,104]. The evolving clinical course of acute cerebral infarction leads to both the unfavorable functional outcome and the survival rate decrease in the post-stroke period [7,8,77]. In our study, patients from EIS group differed in a more severe degree of neurological symptoms at the discharge from the stroke department compared to the group of persons without stroke-in-evolution: 12 (7-42) vs. 3 (2-6) NIHSS score respectively; p < 0.001. Comparison of functional inferiority also confirmed the association of stroke progression with an unsatisfactory functional outcome at the time of leaving the hospital. In patients with EIS, the mRS score reached 4 (3-6) points, without EIS-only 2 (2-3); p < 0.001. Earlier in 2015, we published the results of the survival analysis in 1354 patients during a one-year follow-up after IS. The survival rate in EIS group was only 0.46; 95% CI: 0.42-0.52, while in the group of the favorable clinical course of the stroke it was 0.80; 95% CI: 0.78-0.83; p < 0.001 [14]. Thus, the appropriateness to identify EIS risk factors backed by evidence by the data mentioned above. Put the name of EIS clinical predictors gives physicians the potential to improve the prognosis of stroke by stratifying patients who are at risk of worsening clinical conditions [6,14,49,59,61,104]. Determining independent predictive indicators of EIS based on clinical variables has practical value and ease of implementation. The clinical characteristics used by our research team in constructing the multifactorial tree model are part of the clinical routine and do not require additional laboratory analyzes or instrumental studies. The root node of the decision tree in our study is represented by OCSP stroke classification. In node 1, the tree is divided into two main branches (p < 0.001). The right daughter branch reflects information about patients with POCS, LACS, and PACS stroke subtypes. The left branch represents the data of patients with TACS. This trunk terminates towards the root node 9, in which we can see the distribution of patients with evolving and non-evolving variants of the TACS clinical course. As described above, we initially included in the tree-structured model not only IS subtypes according to the TOAST classification, but also the severity of stroke symptoms on the NIHSS scale. Both of these clinical characteristics represent the clinical manifestations hardness of cerebral infarction. However, when constructing the dendrogram, the conditional inference tree algorithm elected OCSP as the root node, as the variable crucial for creating the predictive model. The German Stroke Study Collaboration has also identified total anterior carotid stroke as the predictor of neurological worsening [66]. Of the 256 patients with IS observed, 13% showed a significant increase in the severity of stroke symptoms in the first 72 hours. The model for predicting the clinical course of stroke was created based on multivariate logistic regression. Territorial infarction, brainstem infarction, diabetes mellitus, internal carotid artery occlusion, and middle cerebral artery (M1) occlusion were identified as the independent predictors of EIS. According to Weimar et al. [66], the recognition of risk factors for stroke worsening using neurological, comorbid, imaging, and ultrasound data allow us to identify patients who could receive the maximum benefit from intensive monitoring of the clinical condition.
In the logistic regression model of other authors [52], the predictors of the progressive clinical course of the cerebral infarction were OCSP classification, the level of conscious, history of coronary heart disease, hyperosmolarity, pyrexia, and the patient’s inhabitation alone. In research of Craig et al. [52], patients with TACS were twice as likely to have EIS as those that had PACS. According to our data, the presence of the total carotid ischemic stroke multiplies the chances of EIS developing compared with the remaining IS subtypes according to OCSP classification (OR 7.78; 95% CI: 5.91-10.23; p<0.001). The univariate analysis of laboratory parameters confirmed the existence of significant differences of the laboratory parameters in EIS. According to our computer dataset, the condition of patients with the evolving brain infarction had obvious signs of the hyperosmolar syndrome: electrolyte imbalance, hyperglycemia, increased levels of nitrogen metabolism in blood plasma. Thus, patients with EIS differed from persons without EIS in such laboratory parameters as sodium (n=1354): 140 (137-143) vs. 139 (136-142)mmol/L; p=0.025; potassium (n=1350): 4.1 (3.8- 4.5) vs. 4.3 (4.0-4.6)mmol/L; p<0.001; glucose (n= 1290): 6.6 (5.7- 8.4) vs. 6.0 (5.2-7.3)mmol/L; p<0.001; creatinine (n=1383): 98 (82-117) vs. 93 (80-110)μmol/L; p < 0.001; and urea (n=1391): 6.5 (5.1-8.7) vs. 6.3 (4.9-7.8)mmol/L; p=0.001. Initially, we introduced into the computed model the values of the bases indicators of the blood chemical profile, such as creatinine, glucose, urea, potassium, and sodium. The urea serum concentration of patients with stroke is presented in the inner node 4. However, in the model of conditional trees, the urea level plays the key role only for predicting the deterioration of patients with PACS and POCS. An increase in urea concentration above 16.6 mmol / L causes branching of the dendrogram to the right, to terminal node 8. When the urea level is ≤ 16.6 mmol / L, the tree extends out to the left, on the inner node 5.
Urea, like creatinine, is an indicator of protein metabolism. Urea is generated in the liver during the neutralization of ammonia formed in the deamination of amino acids. In healthy people, the concentration of urea in the blood plasma corresponds to the range of 2.5 to 8.3mmol/L [105]. Elevation of the urea blood concentration in patients with IS at the admission may be associated with pathological conditions such as impaired renal function, congestive heart failure, extreme loss of salts and fluids, stress, excessive protein catabolism, diabetes mellitus with ketoacidosis [106]. The serum urea level can increase while taking a number of drugs, among which acetylsalicylic acid, diuretics, cephalosporins, and other nephrotoxic medications [107]. The increase in the concentration of urea in blood serum more than 8.5-9.0mmol/L and creatinine more than 117-120mmol/L is indicated by the term “azotemia.” There is absolute azotemia associated with the actual accumulation of residual nitrogenous substances in the blood and relative azotemia due to dehydration [108]. The study of Kwan et al. [81] revealed higher serum urea of patients with EIS compared with patients without EIS: 7.8 vs. 6.5mmol / L; p=0.035. It should be noted that the same authors [81] established the role of total anterior carotid stroke as the risk factor for deterioration in cerebral infarction: 67% vs. 26%, p <0.001. In 2011, Lin et al. [109] put forward the new predictor of EIS, potentially applicable at the admitting level-blood urea nitrogen to creatinine ratio (BUN/ Cr). According to multivariate statistical analysis, it was found that topping up this indicator > 15 almost three and a half times gains the likelihood of IS progressive clinical course. Deng et al. [110] showed that the BUN/Cr ratio is the independent risk factor for hemorrhagic transformation of acute cerebral infarction in patients with diabetes. The researches [110] suggested that nitrogen metabolism indicators increasing with renal insufficiency may be associated with microangiopathy, and, as a consequence, with the development of IS hemorrhagic transformation. In the prospective observational study of Bhatia et al. [58], it was found that hiking up the BUN/Cr ratio>15 and urine specific gravity >1.010 are the independent markers of dehydration in patients with EIS.
In 2012, new data were obtained on the frequency of dehydration in the hospital-admitted stroke patients [111]. The observed cohort of patients with acute cerebrovascular accident included 2,591 people. In 2,158 (83.3%) patients, the ischemic type of the acute vascular brain accident was diagnosed. The laboratory parameters, such as blood urea and creatinine levels, were determined. An increase of the urea/creatinine ratio>80 was considered as the marker of dehydration. It is noteworthy that among patients with acute cerebral infarction, the dehydration rate at the time of hospitalization reached 62.3%. When assessing all types of stroke in general, the dehydration rate was very close to that of IS patients - 61.9% [111]. Lin et al. [93] used the statistical support vector machine algorithms to determine independent predictors of EIS by analyzing the cohort of 382 stroke patients. Levels of urea, sodium, and blood glucose, as potential indicators of dehydration, are included in the prediction equation for the adverse clinical course of cerebral infarction. Up-to-date information about the frequency, predictors, and impact of dehydration on the stroke outcome was published by Cortés-Vicente et al. [112]. Dehydration was determined by the blood urea-to-creatinine ratio >80 and diagnosed in 18 (8.9%) of 203 in-patients. Hypovolemia during hospital stay was significantly associated with the unfavorable stroke outcome at discharge. From the multivariable logistic regression model, the female gender and age were clinical characteristics associated with dehydration [112]. Published studies provide visible evidence of the positive relationship between dehydration of the patients with cerebral infarction and the elevated risk of deep vein thrombosis [113]. Pulmonary embolism due to deep vein thrombosis is the serious complication of stroke, adversely affecting the clinical course and survival of patients [113,114]. Thus, stepping up the values of the nitrogen metabolism indicators, such as urea, creatinine, and the urea-to-creatinine ratio, in the patient with cerebral infarction at the admission, shows not only the state of dehydration but also an increased risk of deep vein thrombosis. Difficult neurological deficiency is an integral part of the clinical presentation of TOAST [19,82]. Immobilization due to hemiplegia and swallowing disturbance also predisposes to the violation of an adequate balance of water and salts in the patient with IS [50,114]. Several researchers recommend satisfactory hydration therapy and anticoagulation as the means of preventing early neurological deterioration in ischemic stroke [50,87,114,115].
Data on the RBC number in the general blood test and IS evolution in a certain way confirm the results above. The patients with the EIS still at the level of the emergency department were characterized by the statistically significant RBC differences: 4.62±0.71 vs. 4.54±0.65 ×1012/L; p = 0.030. Other indicators of the red blood system, such as hemoglobin and hematocrit, did not have remarkable alteration in the compared groups. The proportion of persons with diagnosed anemia (mainly iron deficiency) was 4.2% (18 of 427) for EIS group and 2.2% (22 of 994) for No EIS group (p=0.052). The hematologic system disorders were represented by iron deficiency anemia, polycythemia, essential thrombocytosis, and thrombocytopenia. In the studied cohort of patients (n=1419), there were no persons with sickle cell anemia, aplastic anemia, thalassemia, and malignant blood diseases. We would like to draw attention to the fact that all patients with the hematological disease underwent the consultation with the hematologist or the internist. As it is known, dehydration of IS patients at admission to the clinic is accompanied by the blood viscosity increment [116,117]. The latter circumstance, in turn, is associated with an increased risk of thrombosis and thromboembolic events, such as cerebral infarction [118,119]. Ho’s study [120] convincingly founded the positive relationship between hemoglobin, hematocrit, RBC, and WBC and the state of whole blood viscosity. Previously published data on red blood parameters as the factors of the IS clinical course progression and stroke outcome are controversial [114,116-119,121-124]. Yamamoto et al. [122] performed the risk factors analysis for the cerebral infarction progression by large-artery atherosclerosis. The stroke deterioration rate reached 41.5% (71 out of 171 people). The number of RBC at admission, high-density lipoprotein blood level, degree of arterial stenosis, SBP, heart rate, number of the diffusionpositive lesion on MRI, and NIHSS score presented significant differences in EIS and No EIS groups in univariate data analysis. Lin et al. [123] created the multivariate logistic regression model of EIS based on observation of 2,398 patients with acute stroke. The blood levels of hemoglobin and albumin at admitting were significantly associated with the presence of stroke-in-evolution.
The authors of the publication [123] have suggested that the increased blood viscosity in IS patients leads to stable neurological deterioration during hospitalization. Red blood indicators were compared with the dynamics of the brain infarct growth according to MRI data [124]. MRI was performed twice: before endovascular treatment or intravenous thrombolysis and on the 5th day from the stroke onset. The study showed that the low hemoglobin content in the blood of patients with IS both at the time of hospitalization and after 24 hours is associated with the unsatisfactory functional outcome of treatment. The researchers [124] concluded that the decrease in hemoglobin levels, mainly due to ongoing hemodilution, demonstrates the linear relationship with the final size of the cerebral infarction, as well as with the growth of the ischemic lesion according to neuroimaging data. At the same time, in the cited study [124], there is no data on interrelation of the baseline RBC number with evolving of the cerebral infarction. In previous studies, it was found that the levels of hemoglobin, hematocrit, and the number of RBC match each other [125]. There is explained taking into account their primary physiological function - ensuring sufficient oxygenation of the body [126,127]. When performing an analysis of laboratory data, these parameters are interconnected and, in a certain way, interchangeable [125]. The augmentation in one of these indicators in the general blood test is accompanied, as a rule, by the consensual increase in the other two. That is, in our case, we initially included in the prognostic model two indicators of the red blood system: the number of erythrocytes and the hemoglobin level. However, when constructing the EIS model, including the bifurcation of nodes and expanding the branches of the decision tree, the algorithm chose, as the most suitable cutoff value, the RBC number 4.29 ×1012/L in the general blood test in patients with POCS and PACS.
Currently, there are several algorithms for creating trees based on various logical principles of construction and optimization criteria for the predictive models [128]. In 2016, our research team published the results of determining independent risk factors for progressive IS using the decision tree method [13]. To solve the classification problems, we used the statistical algorithm of Classification and Regression Trees (CART) [32,129]. According to the calculations, the prognostic model included several independent predictors: OCSP stroke subtype, initial NIHSS score, the blood glucose level on admission, and congestive heart failure. The accuracy of the classification of 1421 patients into progressive and regressive IS groups according to the CART algorithm was 0.78; 95% CI: 0.76-0.80, sensitivity-0.47, specificity-0.92 [13]. In the present article, we used in principle different approach for computing the decision tree-the conditional inference trees algorithm [38,39]. The indicated mechanism for statistical testing hypotheses takes into account the nature of the distribution of the independent variables and, at each step of the recursive data partitioning, selects the unbiased set of predictors using the formal test based on the statistical criterion (criterion-Figure 2). The estimation of statistical significance (significance - Figure 2) of this criterion is carried out arranging the permutation test [128]. As a result of the conditional tree algorithm implementation, the excessive complication of the tree-structured model is prevented. In the example of Figure 1, we can see the compact decision tree of EIS that does not require the pruning procedure.
Limitations
We look back on some limitations of our investigation. Of course, due to the randomly selected cohort of patients, there may be a pick bias. The research team effort to lessen this issue by enlargement the number of patients enrolled in the study until 1421 persons with acute IS. The evolving course of the stroke is defined as the increase in neurological symptoms severity by ≥2 NIHSS points. In contemporary research evaluating the results of endovascular treatment and intravenous thrombolysis, the EIS is considered to be the deterioration of the neurological condition by 4 or more points on the NIHSS scale [50,130]. The previous version of the Belarusian national clinical protocol, dictating the conditions and procedure for providing medical care to patients with cerebral infarction in 2005-2017, did not include intravenous administration of rtPA and endovascular treatment [22]. Performing thrombolysis in acute IS was rare and sporadic [77,131]. This circumstance served as the basis for not including IS patients with reperfusion therapy in our computer database. When analyzing the dataset, we did not take into account swallowing disorders, since this was not initially included in the study protocol. In the Republic of Belarus, there is not yet the sufficient number of qualified specialists who can adequately assess dysphagia in stroke and implement effective methods of rehabilitation for this complication [131]. Unfortunately, we were not able to perform the detailed analysis of hemorheological parameters using new technologies, such as viscoelastic methodologies and scanning electron microscopy approach [132]. The standard assessment of the patient condition using specialized stroke scales was not an obligatory part of the routine work of the neurologist [131]. Although the population of the Republic of Belarus currently does not reach 10 million people [1], there is no single national stroke register [21,77,131]. Belarus also does not participate in any of the international cooperative registers of thrombolysis [131].
The first author of the presented article (IG), while working in the healthcare system of the Republic of Belarus, on behalf of the Ministry of Health has headed and coordinated the activities of the group of Belarusian specialists in the field of neurology (2015-2017) [21,77]. The result of this work was the novel clinical national protocol, which entered into force in January 2018 throughout the republic [133]. The new protocol is based on international recommendations for the treatment of neurological diseases. In terms of helping patients with IS, the protocol contains endovascular treatment and intravenous thrombolysis with rtPA, assessment conditions according to specialized stroke scales, the using of thenon-vitamin K antagonist oral anticoagulants for cardioembolic IS by nonvalvular AF, adequate measures for the correction of dehydration, as well as help for the patients with EIS [133].
Conclusion
The presented work is devoted to the search for patterns of the evolving clinical course of IS in 1421 patients with acute stroke. According to the results of the univariate data analysis, 21 clinical, demographic, laboratory variables accommodated in the computer database were included in the conditional inference trees statistical algorithm. The prognostic statistical model of EIS has been constructed. Thereby, such independent predictors of evolving IS having been identified: the stroke subtype according to OCSP classification, the serum urea level and RBC number in the total blood count. The accuracy of the statistical model reaches 0.77; 95% CI: 0.75-0.80. The sensitivity is 0.52, the specificity - 0.88, PPV-0.66, NPV-0.81; p < 0.001.
Author Contributions
IG participated in the organization of the scientific project, creating the study design, data gathering, and extraction, statistical analysis, drafting title, abstract, abbreviation, text, tables, figures, references, data interpretation. Igor Prudyvus carried out statistical analysis, figures, and search strategy design. Both authors have reviewed and approved the final manuscript version.
Acknowledgments
IG was employed by the research associate, senior research associate, and principal research associate in the department of neurology at the RSPCNN (2002-2015). She later served as the deputy director of the same institution (2015-2017). Since 2017, IG has been living in the United States. IG previously led five research projects on IS. The source of financing for the projects was the funds allocated by the State Committee for Science and Technology of the Republic of Belarus. Detailed information about the projects is displayed on the ORHID website: https://orcid.org/0000-0001- 6648-1589. Therefore, the entire computer dataset of patients with IS, research design, and protocol, the theoretical and practical framework of the study was originated by IG. The project was implemented as part of her doctoral dissertation “Cardiovascular predictors of clinical course, functional outcome and survival of patients with ischemic stroke” in the specialty “Neurological disorders.”
References
- World Bank Open Data (2018) World Bank Group. Country profile: Belarus, Europe.
- GBD 2016 Lifetime Risk of Stroke Collaborators, Feigin VL, Nguyen G, Cercy K, Johnson CO, et al. (2018) Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 379(25): 2429-2437.
- GBD 2016 Stroke Collaborators (2019) Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 18: 459-480.
- Dávalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, et al. (1999) Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke 30(12): 2631-2636.
- Fisher M, Garcia JH (1996) Evolving stroke and the ischemic penumbra. Neurology 47(4): 884-888.
- Geng HH, Wang Q, Li B, Cui BB, Jin YP, et al. (2017) Early neurological deterioration during the acute phase as a predictor of long-term outcome after first-ever ischemic stroke. Medicine (Baltimore) 96(51): e9068.
- Gontschar I (2011) Heart rate variability state in patients with progressive atherothrombotic ischemic stroke (in Russian). Far Eastern Medical Journal 2: 12-15.
- Gontschar IA, Stepanova JI, Prudyvus IS (2013) Biochemical predictors and markers of ischemic stroke (in Russian). In VS Kamyschnikov (Ed.), Belarusian Medical Academy of Postgraduate Education, Belarus, Europe, pp. 1-512.
- Caplan LR (2002) Worsening in ischemic stroke patients: Is it time for a new strategy? Stroke 33(6): 1443–1445.
- Bogousslavsky J, Van Melle G, Regli F (1988) The Lausanne stroke registry: analysis of 1,000 consecutive patients with first stroke. Stroke 19(9): 1083-1092.
- Yamamoto H, Bogousslavsky J, van Melle G (1998) Different predictors of neurological worsening in different causes of stroke. Arch Neurol 55(4): 481-486.
- Moulin T, Tatu L, Vuillier F, Berger E, Chavot D, et al. (2000) Role of a stroke data bank in evaluating cerebral infarction subtypes: patterns and outcome of 1,776 consecutive patients from the besançon stroke registry. Cerebrovasc Dis 10(4): 261-271.
- Gontschar IA, Prudyvus IS (2016) Prediction of progressive clinical course of ischemic stroke by dendrogramm method (in Russian). Meditsinskienovosti 7: 67-70.
- Gontschar IA, Prudyvus IS, Nedzvedz GK (2015) Progressive ischemic stroke: functional outcome and survival (in Russian). Meditsinskienovosti 2: 68-71.
- WHO Monica Project Investigators (1988) The world health organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol 41(2): 105-114.
- Liden PD. NIH Stroke Scale/ Score (NIHSS). MDCalc.
- Banks JL, Marotta CA (2007) Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 38(3): 1091-1096.
- World Medical Association (WMA) (2018) Declaration of Helsinki - Ethical principles for medical research involving human subjects. 2019 World Medical Association, France.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337(8756): 1521-1526.
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, et al. (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24(1): 35-41.
- Gontschar I, Prudyvus I (2019) The computer database of 1421 Belarusian patients with ischemic stroke: Design, methods, baseline characteristics, and evolving clinical course. EC Neurology.
- Clinical Protocols for the diagnosis and treatment of patients with pathology of the nervous system (in Russian). Standards of diagnostic and treatment (2005) Ministry of Health of the Republic of Belarus.
- Murdoch D (2016) Download R 3.2.5 for Windows (64 megabytes, 32/64 bit).
- Rickert J, Balasubramanian N, Kane M (2018) Conference report: R/medicine report. The R Journal 10(2): 581-582.
- Flach PA (2004) The many faces of ROC analysis in machine learning. The twenty-first international conference on machine learning. University of Bristol, UK.
- Fox J, Weisberg S (2011) An R companion to applied regression (2nd edn). Thousand Oaks 22(2): 418-419.
- Baier T, Neuwirth E (2007) Excel:: COM :: R. Computational Statistics 22(1): 91-108.
- Ford C (2015) Understanding Q-Q plots. Research Data Services + Sciences, University of Virginia Library, U S.
- Gross J, Ligges U (2015) Package ‘nortest’. Tests for normality pp 1-10.
- Freeman E (2012) Package ‘presenceabsence’. Presence-Absence model evaluation, pp. 1-40.
- Sarkar Deepayan (2008) Lattice: Multivariate data visualization with R. Springer Publishing Company, Inc.
- Sing T, Sander O, Beerenwinkel N, Lengauer T (2015) Package ‘ROCR’. Visualizing the performance of scoring classifiers 1-14.
- Venables WN, Smith DM, and R Core Team (2019) An Introduction to R. Notes on R: A programming environment for data analysis and graphics 1-99.
- Chongsuvivatwong V (2008) Analysis of epidemiological data using R and Epicalc. Prince of Songkla University, Thailand 1-313.
- Zou KH, O Malley AJ, Mauri L (2007) Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 115(5): 654-657.
- Gontschar IA (2011) Prognostic model construction of non-cardioembolic ischemic stroke progression using discriminant analysis. Meditsinskienovosti 1: 69-76.
- Le J (2018) Decision trees in R. Machine learning.
- Hothorn T, Hornik K, Zeileis A (2006) Unbiased recursive partitioning: A conditional inference framework. Journal of Computational and Graphical Statistics 15(3): 651-674.
- Hothorn T, Hornik K, Zeileis A (2019) Conditional inference procedures in a permutation test framework.
- Strasser H, Weber C (1999) On the asymptotic theory of permutation statistics. Department of statistics, Vienna University of Economics and Business Administration, Austria.
- Therneau TM, Atkinson B (2019) Package ‘rpart’: Recursive Partitioning and Regression Trees, 1-33.
- Sardá A, Subbiah S, Bartz T (2017) Conditional inference trees for knowledge extraction from motor health condition data. Engineering Applications of Artificial Intelligence 62: 26-37.
- Wu Z, Su X, Sheng H, Chen Y, Gao X, et al. (2017) Conditional inference tree for multiple gene-environment interactions on myocardial infarction. Arch Med Res 48(6): 546-552.
- König HH, Leicht H, Bickel H, Fuchs A, Gensichen J, et al. (2013) Effects of multiple chronic conditions on health care costs: an analysis based on an advanced tree-based regression model. BMC Health Serv Res 13: 219.
- Zhang T, Fulk GD, Tang W, Sazonov ES (2013) Using decision trees to measure activities in people with stroke. Conf Proc IEEE Eng Med Biol Soc pp. 6337-6340.
- Ishwaran H, Lu M (2019) Standard errors and confidence intervals for variable importance in random forest regression, classification, and survival. Stat Med 38(4): 558-582.
- Williams B, Mancia G, Spiering W, AgabitiRosei E, Azizi M, et al. (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: The task force for the management of arterial hypertension of the European society of cardiology and the european society of hypertension. J Hypertens 36(10): 1953-2041.
- Mrochek AG, Netschesova TA, Korobko IY, Liventseva MM, Pavlova OS, et al. (2010) Arterial hypertension: prevention, diagnostics and management. National guidelines.
- Yi X, Lin J, Wang Y, Zhou J, Zhou Q, et al. (2018) Response to clopidogrel is associated with early neurological deterioration after acute ischemic stroke. Oncotarget 9(28): 19900-19910.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, et al. (2018) 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3): e46-e110.
- Miyamoto N, Tanaka Y, Ueno Y, Kawamura M, Shimada Y, et al. (2013) Demographic, clinical, and radiologic predictors of neurologic deterioration in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 22(3): 205-210.
- Craig LE, Wu O, Gilmour H, Barber M, Langhorne P (2011) Developing and validating a predictive model for stroke progression. Cerebrovasc Dis Extra 1(1): 105-114.
- Siegler JE, Boehme AK, Albright KC, George AJ, Monlezun DJ, et al. (2013) A proposal for the classification of etiologies of neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis 22(8): e549-e556.
- Millikan CH, Siekert RG (1955) Studies in cerebrovascular disease. I. The syndrome of intermittent insufficiency of the basilar arterial system. Proc Staff Meet Mayo Clin 30(4): 61-68.
- Millikan CH, Siekert RG (1955) Studies in cerebrovascular disease. IV. The syndrome of intermittent insufficiency of the carotid arterial system. Proc Staff Meet Mayo Clin 30(9): 186-191.
- Thanvi B, Treadwell S, Robinson T (2008) Early neurological deterioration in acute ischaemic stroke: Predictors, mechanisms and management. Postgrad Med J 84(994): 412-417.
- Fukuoka T, Nakazato Y, Kawasaki H, Ikeda K, Furuya T, et al. (2018) The clinical features of ischemic stroke patients for whom smoking was considered the sole risk factor for ischemic stroke. Intern Med 57(12): 1703-1706.
- Bhatia K, Mohanty S, Tripathi BK, Gupta B, Mittal MK, et al. (2015) Predictors of early neurological deterioration in patients with acute ischaemic stroke with special reference to blood urea nitrogen (BUN)/creatinine ratio & urine specific gravity. Indian J Med Res 141(3): 299-307.
- Nacu A, Bringeland GH, Khanevski A, Thomassen L, Waje‐Andreassen U, et al. (2016) Early neurological worsening in acute ischaemic stroke patients. Acta Neurol Scand 133(1): 25-29.
- Toni D, Fiorelli M, Gentile M, Bastianello S, Sacchetti ML, et al. (1995) Progressing neurological deficit secondary to acute ischemic stroke. A study on predictability, pathogenesis, and prognosis. Arch Neurol 52(7): 670-675.
- Chang Y, Kim J, Kim MH, Kim YJ, Song TJ, et al. (2018) Interarm blood pressure difference is associated with early neurological deterioration, poor short-term functional outcome, and mortality in noncardioembolic stroke patients. J Clin Neurol 14(4): 555-565.
- Siegler JE, Boehme AK, Kumar AD, Gillette MA, Albright KC, et al. (2013) Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis 22(7): e207-e213.
- Bang OY, Kim GM, Chung CS, Kim SJ, Kim KH, et al. (2010) Differential pathophysiological mechanisms of stroke evolution between new lesions and lesion growth: perfusion-weighted imaging study. Cerebrovasc Dis 29(4): 328-335.
- Kim H, Kim Y, Kim YW, Kim SR, Yang SH, et al. (2016) Perfusion weighted MRI parameters for prediction of early progressive infarction in middle cerebral artery occlusion. J Korean NeurosurgSoc 59(4): 346-351.
- Huang YC, Tsai YH, Lee JD, Yang JT, Pan YT, et al. (2018) A novel neuroimaging model to predict early neurological deterioration after acute ischemic stroke. Curr Neurovasc Res 15(2): 129-137.
- Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, et al. (2005) Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol 62(3): 393-397.
- Lee SJ, Hong JM, Lee SE, Kang DR, Ovbiagele B, et al. (2017) Association of fibrinogen level with early neurological deterioration among acute ischemic stroke patients with diabetes. BMC Neurol 17(1): 101.
- Baizabal Carvallo JF, Juarez AM, Samson Y (2014) Clinical deterioration following middle cerebral artery hemodynamic changes after intravenous thrombolysis for acute ischemic stroke. J Stroke Cerebrovasc Dis 23(2): 254-258.
- Seners P, Turc G, Tisserand M, Legrand L, Labeyrie MA, et al. (2014) Unexplained early neurological deterioration after intravenous thrombolysis: Incidence, predictors, and associated factors. Stroke 45(7): 2004-2009.
- Zhang YB, Su YY, He YB, Liu YF, Liu G, et al. (2018) Early neurological deterioration after recanalization treatment in patients with acute ischemic stroke: A retrospective study. Chin Med J (Engl) 131(2): 137-143.
- Lee SJ, Hwang YH, Hong JM, Choi JW, Yoon BS, et al. (2018) Impact of varying levels of hyperglycemia on clinicoradiographic outcomes after endovascular reperfusion treatment. Sci Rep 8(1): 9832.
- Helleberg BH, Ellekjær H, Rohweder G, Indredavik B (2014) Mechanisms, predictors and clinical impact of early neurological deterioration: the protocol of the trondheim early neurological deterioration study. BMC Neurol 14: 201.
- Zhang Z, Pu Y, Mi D, Liu L (2019) Cerebral hemodynamic evaluation after cerebral recanalization therapy for acute ischemic stroke. Front Neurol 10: 719.
- Hou X, Chen W, Xu H, Zhu Z, Xu Y, et al. (2019) The rate of early neurological deterioration occurring after thrombolytic therapy: A meta-analysis. Brain Behav 9(2): e01210.
- Edwards DN, Bix G (2019) Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am J Physiol Cell Physiol 316(2): C252-C263.
- Backhaus R, Boy S, Fuchs K, Ulrich B, Schuierer G, et al. (2013) Hyperperfusion syndrome after MCA embolectomy-A rare complication? Am J Case Rep 14: 513-517.
- Gontschar I, Prudyvus I (2019) Clinical prognostic factors for one-year survival in patients after ischemic stroke. Res & Rev Neurosci 3(1): 1-11.
- Caplan LR (2011) Stroke classification: A personal view. Stroke 42(1 Suppl): S3-S6.
- Mohr JP, Caplan LR, Melski JW, Goldstein RJ, Duncan GW, et al. (1978) The Harvard cooperative stroke registry: A prospective registry. Neurology 28(8): 754-762.
- Mazya MV, Cooray C, Lees KR, Toni D, Ford GA, et al. (2018) Minor stroke due to large artery occlusion. When is intravenous thrombolysis not enough? Results from the SITS International Stroke Thrombolysis Register. Eur Stroke J 3(1): 29-38.
- Kwan J, Hand P (2006) Early neurological deterioration in acute stroke: Clinical characteristics and impact on outcome. QJM 99(6): 625-633.
- Tei H, Uchiyama S, Ohara K, Kobayashi M, Uchiyama Y, et al. (2000) Deteriorating ischemic stroke in 4 clinical categories classified by the oxfordshire community stroke project. Stroke 31(9): 2049-2054.
- Paci M, Nannetti L, D'Ippolito P, Lombardi B (2011) Outcomes from ischemic stroke subtypes classified by the oxfordshire community stroke project: A systematic review. Eur J PhysRehabil Med 47(1): 19-23.
- Siegler JE, Samai A, Semmes E, Schild MS (2016) Early neurologic deterioration after stroke depends on vascular territory and stroke etiology. J Stroke 18(2): 203-210.
- Alexander P, Heels Ansdell D, Siemieniuk R, Bhatnagar N, Chang Y, et al. (2016) Hemicraniectomy versus medical treatment with large MCA infarct: A review and meta-analysis. BMJ Open 6(11): e014390.
- Kumar AD, Boehme AK, Siegler JE, Gillette M, Albright KC, et al. (2013) Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrovasc Dis 22(7): e111-e117.
- Lin J, Weng Y, Li M, Mo Y, Zhao J (2018) Hydration prevents chronic hyperglycaemic patients from neurological deterioration post-ischaemic stroke. Acta Neurol Scand 137(6): 557-565.
- Kwon HM, Lee YS, Bae HJ, Kang DW (2014) Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke 45(3): 871-873.
- Barber M, Langhorne P, RumleyA, Lowe GD, Stott DJ (2006) D-dimer predicts early clinical progression in ischemic stroke: Confirmation using routine clinical assays. Stroke 37(4): 1113-1115.
- Zang R, Zhang H, Xu Y, Zhang S, Liu X, et al. (2016) Serum c-reactive protein, fibrinogen and D-dimer in patients with progressive cerebral infarction. Translational Neuroscience 7(1): 84-88.
- Vila N, Castillo J, Dávalos A, Chamorro A (2000) Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31(10): 2325-2329.
- Kim TJ, Kang MK, Jeong HG, Kim CK, Kim Y, et al. (2017) Cystatin c is a useful predictor of early neurological deterioration following ischaemic stroke in elderly patients with normal renal function. Eur Stroke J 2(1): 23-30.
- Lin J, Jiang A, Ling M, Mo Y, Li M, et al. (2018) Prediction of neurologic deterioration based on support vector machine algorithms and serum osmolarity equations. Brain Behavior 8(7): e01023.
- Martin AJ, Price CI (2018) A systematic review and meta-analysis of molecular biomarkers associated with early neurological deterioration following acute stroke. Cerebrovascular Diseases 46(5-6): 230-241.
- AlexandrovAV, Felberg RA, Demchuk AM, Christou I, Burgin WS, et al. (2000) Deterioration following spontaneous improvement: sonographic findings in patients with acutely resolving symptoms of cerebral ischemia. Stroke 31(4): 915-919.
- You W, Li Y, Ouyang J, Li H, Yang S, et al. (2019) Predictors of poor outcome in patients with minor ischemic stroke by using magnetic resonance imaging. J Mol Neurosci 2019 Jul 20 USA.
- Boulouis G, Lauer A, Siddiqui AK, Charidimou A, Regenhardt RW, et al. (2017) Clinical imaging factors associated with infarct progression in patients with ischemic stroke during transfer for mechanical thrombectomy. JAMA Neurol 74(11): 1361-1367.
- RenúJornet A, Urra X, Laredo C, Montejo C, Rudilosso S, et al. (2019) Benefit from mechanical thrombectomy in acute ischemic stroke with fast and slow progression. J Neurointerv Surg 2019 Jul 4 USA.
- Lin CJ, Tsai YY, Hsiao KY, Tsai YH, Lee MH, et al. (2017) Urine-specific gravity-based hydration prevents stroke in evolution in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 26(9): 1885-1891.
- Zhang C, Zhao S, Zang Y, Gu F, Mao S, et al. (2017) The efficacy and safety of dl-3n-butylphthalide on progressive cerebral infarction: a randomized controlled strobe study. Medicine (Baltimore) 96(30): 5835-5840.
- Kanamaru T, Suda S, Muraga K, Okubo S, Watanabe Y, et al. (2017) Albuminuria predicts early neurological deterioration in patients with acute ischemic stroke. J Neurol Sci 372: 417-420.
- Birchel P, Ellul J, Barer D (2004) Progressing stroke: towards an internationally agreed definition. Cerebrovasc Dis 17(2-3): 242-252.
- Barber M, Wright F, Stott DJ, Langhorne P (2004) Predictors of early neurological deterioration after ischaemic stroke: a case-control study. Gerontology 50(2): 102-109.
- Yi X, Lin J, Li J, Zhou Q, Han Z (2017) Epoxyeicosatrienoic acids are mediated by EPHX2 variants and may be a predictor of early neurological deterioration in acute minor ischemic stroke. J Atheroscler Thromb 24(12): 1258-1266.
- Lelevich VV, Vorobyov VV, Grinevich TN (2011) Clinical laboratory diagnostics: teaching aid (in Russian). Grodno State Medical University, Grodno, Belarus 1-166.
- Bowker LK, Briggs RS, Gallagher PJ, Robertson DR (1992) Raised blood urea in the elderly: a clinical and pathological study. Postgrad Med J 68(797): 174-179.
- De Vecchis R, Ciccarelli A, Ariano C, Cioppa C, Giasi A, et al. (2011) In chronic heart failure with marked fluid retention, the i.v. high doses of loop diuretic are a predictor of aggravated renal dysfunction, especially in the set of heart failure with normal or only mildly impaired left ventricular systolic function. Minerva Cardioangiol 59(6): 543-554.
- Kirpichenok LN, Skreblo EI, Shilin VE, Tihon TV (2012) Workshop on clinical laboratory diagnostics: manual (in Russian). Vitebsk State Medical University, Vitebsk, Belarus 1-176.
- Lin LC, Yang JT, Weng HH, Hsiao CT, Lai SL, et al (2011) Predictors of early clinical deterioration after acute ischemic stroke. Am J Emerg Med 29(6): 577-581.
- Deng L, Qiu S, Wang C, Bian H, Wang L, et al (2019) Effects of the blood urea nitrogen to creatinine ratio on haemorrhagic transformation in AIS patients with diabetes mellitus. BMC Neurol 19(1): 63.
- Rowat A, Graham C, Dennis M (2012) Dehydration in hospital-admitted stroke patients: detection, frequency, and association. Stroke 43(3): 857-859.
- Cortés-Vicente E, Guisado-Alonso D, Delgado-Mederos R, Camps-Renom P, Prats-Sánchez L, et al. (2019) Frequency, risk factors, and prognosis of dehydration in acute stroke. Front Neurol 10: 305.
- Kelly J, Hunt BJ, Lewis RR, Swaminathan R, Moody A, et al. (2004) Dehydration and venous thromboembolism after acute stroke. Q J Med 97(5): 293-296.
- Adams HP,Del Zoppo G, Alberts MJ, Bhatt DL, Brass L, et al. (2007) Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council and the atherosclerotic peripheral vascular disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 115(20): e478-534.
- Khan MT, Ikram A, Saeed O, Afridi T, Sila CA, et al. (2017) Deep vein thrombosis in acute stroke - A systemic review of the literature. Cureus 9(12): p.e1982.
- Bahouth MN, Gottesman RF, Szanton SL (2018) Primary dehydration and acute stroke: A systematic research review. J Neurol 265(10): 2167-2181.
- Furukawa K, Abumiya T, Sakai K, Hirano M, Osanai T, et al. (2016) Increased blood viscosity in ischemic stroke patients with small artery occlusion measured by an electromagnetic spinning sphere viscometer. J Stroke Cerebrovasc Dis 25(11): 2762-2769.
- Yang R, Wang A, Ma L, Su Z, Chen S, et al. (2018) Hematocrit and the incidence of stroke: a prospective, population-based cohort study. Ther Clin Risk Manag 14: 2081-2088.
- Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, et al. (2013) Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44(3): 870-947.
- Ho CH (2004) White blood cell and platelet counts could affect whole blood viscosity. J Chin Med Assoc 67(8): 394-397.
- Lee C-H, Jung K-H, Cho DJ, Jeong S-K (2019) Effect of warfarin versus aspirin on blood viscosity in cardioembolic stroke with atrial fibrillation: A prospective clinical trial. BMC Neurology 19(1): 82.
- Yamamoto N, Satomi J, Yamamoto Y, Shono K, Kanematsu Y, et al. (2017) Risk factors of neurological deterioration in patients with cerebral infarction due to large-artery atherosclerosis. J Stroke Cerebrovasc Dis 26(8): 1801-1806.
- Lin LC, Lee TH, Chang CH, Chang YJ, Liou CW, et al. (2012) Predictors of clinical deterioration during hospitalization following acute ischemic stroke. Eur Neurol 67(3): 186-192.
- Bellwald S, Balasubramaniam R, Nagler M, Burri MS, Fischer SDA, et al. (2018) Association of anemia and hemoglobin decrease during acute stroke treatment with infarct growth and clinical outcome. PLoS One 13(9):
- Tefferi A, Hanson CA, Inwards DJ (2005) How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc 80(7): 923-936.
- Grotta JC, Manner C, Pettigrew LC, Yatsu FM (1986) Red blood cell disorders and stroke. Stroke 17(5): 811-817.
- Tang B, Yu X, Xu L, Zhu A, Zhang Y, et al. (2019) Continuous noninvasive hemoglobin monitoring estimates timing for detecting anemia better than clinicians: A randomized controlled trial. BMC Anesthesiol 19(1): 80.
- Shitikov VK, Mastitsky SE (2017) Classification, regression and other data mining algorithms using R. London: 1351.
- Breiman L, Friedman JH, Olshen RA, Stone CJ (1984) Classification and regression trees (the wadsworth statistics/probability series). Wadsworth Publishing 1-358.
- Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, et al. (2003) Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34(8): e109-e137.
- Farrington J, Pezzella FR, Yakovlev A, Rotar O (2017) Review of acute care and rehabilitation services for heart attack and stroke in Belarus. WHO Regional Office for Europe pp. 1-42.
- De Villiers S, Swanepoel A, Bester J, Pretorius E (2016) Novel diagnostic and monitoring tools in stroke: an individualized patient-centered precision medicine approach. J Atheroscler Thromb 23(5): 493-504.
- Clinical Protocol: Diagnosis and treatment of patients with diseases of the nervous system (adult population) (2018). Standard of diagnostic and treatment. Ministry of Health of the Republic of Belarus, Russian.
© 2019 Irina Gontschar. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






