- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Quantitative Analysis of Electroencephalographic Background Activity in NREM Sleep in Childhood Epilepsy with Centrotemporal Spikes
Vinţan Mihaela Adela1* and Muresanu Fior Dafin2
1Neuroscience Department, Romania
2Head of Neuroscience Department, Romania
*Corresponding author: Vintan Mihaela Adela, University Assistant Pediatric Neurology, Neuroscience Department, University of Medicine and Pharmacy Iuliu Hatieganu, Cluj-Napoca, Romania
Submission: August 22, 2019;Published: September 13, 2019

ISSN 2637-7748
Volume2 Issue5
Abstract
Background: Childhood epilepsy with centrotemporal spikes (also known as Rolandic epilepsy) is one of the most frequent epileptic syndromes in childhood. Diagnosis is based on typical seizures and electroencephalographic features:
symmetric background EEG activity, well organized, with normal reactivity in wakefulness and normal sleep patterns;
interictal rolandic epileptiform discharges. Quantitative analysis of the EEG recording (QEEG) is a way of assessing the brain’s electrical activity. Compared with analog EEG, it provides additional data in neurological disorders where no structural brain damage is present, but rather an alteration of how the brain evaluates information. In epilepsy, QEEG studies are limited.
Objectives: To identify whether there are present changes in background activity, using quantitative analysis of sleep EEG, routinely obtained in the diagnostic stages, compared with a group of healthy children.
Method and subjects: We performed an observational, transversal, case-control type study, that included two groups: rolandic epilepsy patients and a control group of comparable age.
Result: Relative powers were obtained for delta (0.5-3.5Hz), theta (4.0-7.5Hz), alpha (8.0-12.5Hz) and beta (13.0-30.0Hz) band frequencies, for the electrodes: FP1, FP2, F7, F8, C3, C4, T5, T6, P3, P4, O1, O2. Dominant relative power was delta band in both groups, 73.58-77.79% in the epilepsy group, and 68.11 - 78.37% in the control group, with maximum at the occipital electrodes (epilepsy group); occipital, central and frontal (control). Comparison of average delta relative power between two groups, using t test for independent samples, showed no significant differences, except for the C3 electrode (t=2.12, p<0.05), with delta relative power higher for epilepsy patients. There were no significant differences between left and right hemispheric relative power of delta, theta and alpha frequencies in epilepsy group. In terms of relative power for beta frequency, it is a dominance for the right hemisphere for pairs of central electrodes C3/C4 (1.42/1.55, t=2.32, p<0.05). Amplitudes of the background activity, in each electrode, were evaluated and compared between control and epilepsy group-for sequences provided by quantitative processing program, respectively: 9.131sec, 9.622sec, 10.132sec and 10.623sec. there were no significant differences.
Keywords: Epilepsy; Rolandic; Centrotemporal; Children; QEEG
Abbreviations: BRE: Benign Rolandic Epilepsy; NREM: Non rapid eyes movements sleep; REM: Rapid eyes movement sleep; EEG: Electroencephalography; QEEG: Quantitative EEG; ADHD: Attention-Deficit/ Hyperactivity disorder
Introduction
Childhood epilepsy with centrotemporal spikes, also known as benign childhood epilepsy with centrotemporal spikes (BCECTS) or benign rolandic epilepsy (BRE) is a self-limiting epileptic syndrome and the most common epilepsy in children [1]. It is characterized by the presence of typical rolandic seizures and electroencephalographic changes that are characteristic and diagnostic [1-5]. EEG background activity is symmetrical, well organized, with normal reactivity in awake state, and normal sleep patterns. Physiological elements of sleep and cyclic organization are retained even in the presence of seizures [5], interictal epileptiform discharge-rolandic characteristic spikes. There are described cases with focal slow activity, episodic, unilateral or bilateral [6,7]. BRE seizures occur mainly during sleep, it is considered that intracortical inhibition is decreased in motor cortex during NREM sleep in patients with frontal lobe epilepsy, that is the case of BRE children [8].
Quantitative analysis of the electroencephalographic activity (QEEG), shows subtle changes in frequency and coherence abnormalities in brain activity, between different brain regions. Quantitative EEG (QEEG, topographic EEG, or brain electrical activity mapping-BEAM) allows revealing of location and extension of brain dysfunction in specific frequency bands and in special conditions. QEEG is an effective way for analysis of structural and functional brain pathology [9-12]. QEEG can detect abnormalities in early stages of dementia, is sensitive in differentiating between depression and dementia. There are QEEG studies in postcommotional chronic syndrome diagnosis, [13,14]. QEEG was used to assess brain electrical activity in hypoxic-ischemic type sufferance in an immature brain: studies in neonates with hypoxicischemic encephalopathy or in children after open heart surgery [15]. Changes in EEG power spectra are important diagnostic factors for ADHD and autism diagnosis. QEEG studies in epilepsy are limited. There were evaluated changes in temporal epilepsy with hippocampal sclerosis. In other forms of epilepsy in children, QEEG studies are few and are mainly related to the identification and analysis of epileptiform discharges.
Method and Subjects
1.1. Objectives
The purpose of the study was to identify if there are present any changes in EEG background activity, using quantitative analysis of the background activity in NREM sleep. The study included rolandic epilepsy, at the onset of seizures, before treatment, and is compared with a group of healthy children. Quantitative analysis was performed with the software equipment of EEG recording device.
1.2. Subjects
We conducted an observational study, transverse, case-control type, which included children with rolandic epilepsy (BRE) monitored in Clinic of Pediatric Neurology from 2004 to 2010 and a control group of comparable age. BRE group included 20 children, mean age 7.38±2.66 years (ages 3-14 years), 13 boys, 7 girls.
I. Inclusion criteria
A. absence of cognitive impairment (Raven Progressive Matrices),
B. normal neurological examination,
C. clinically normal hearing, no history of hearing damage,
D. normal visual acuity-clinical, no history of visual disturbances.
II. Exclusion criteria
A. abnormalities of hearing-identified clinically or by history,
B. visual anomalies-identified clinically or by history.
Control group-included 15 children who met the inclusion and exclusion criteria, mean age 7.93 ± 3.01 (aged 5-13 years), 3 boys, 12 girls. Groups did not meet equivalence in relation with patient sex, there are studies reporting no differences between the sexes in terms of EEG in NREM sleep [16]. Inclusion criteria: 1) children without symptoms and/or neurological signs when assessing, 2) absence of personal history of neurological sufferance (seizures, TBI, personal history of perinatal distress), 3) absence of family neurological history (epilepsy or isolated seizures). Exclusion criteria: 1) presence of signs/criteria for ADHD, 2) Clinical signs of autism or autistic-like disorders, 3) abnormalities on EEG recording, 4) abnormalities of hearing - clinical or history; 5) visual abnormalities - clinical or history.
1.3. Method
For both groups following evaluations were performed:
A. personal history-data ante-/peri-neonatal period, assessment of psychomotor development at age stages.
B. age-adjusted neurological examination.
C. clinical evaluation of auditory and visual acuity.
D. psychiatric examination.
E. psychological testing-Raven progressive matricesstandardized instrument designated for measuring overall intelligence, adjusted for Romanian population, ADHD scales.
F. computerized neuropsychological evaluation using CANTAB battery of tests.
G. digital EEG recording-recording of brain electrical activity with 19 Ag/AgCl surface electrodes, filter between 0.5Hz and 35Hz. Signal amplification was done with a Brain Quick System 1998 equipment. Impedance was kept below 10kΩ. Electrode placement was done according to the international 10/20, plus a vertex reference electrode. The examination was during the night sleep, recording time between 2 and 4 hours. There was no previous sleep deprivation. No medication was administered to induce sleep, or other medication. BRE patients were before the establishment antiepileptic therapy. Recordings were made with the child supine, in casual attire, in noise-free environment, with low light. We performed visual analysis of each recording noting the background activity, motion artifacts, stages of sleep and epileptiform discharge.
H. Quantitative EEG (QEEG)-using the program of quantitative analysis of Brain Quick System equipment. We selected 25 to 30 epocs from sleep EEG recording, lasting 2 seconds. In epocs selection were excluded artifacts areas. Epocs selection was done in 2 and 3 stages of sleep, defined by the presence of sleep spindles and K complexes. In epocs selection in BRE group, epileptiform discharges were excluded. After applying Fourier transformation, we studied relative power of frequency bands: delta (0.5-3.5Hz), theta (4.0-7.5Hz), alpha (8.0-12.5Hz) and beta (13.0-30.0Hz). We obtained relative power amplitude, the software used calculated sequences of 9131sec, 9622sec, 10.132 seconds and 10.632 seconds respectively. The analysis was performed for the following 12 electrodes: FP1, FP2, F7, F8, C3, C4, T5, T6, P3, P4, O1, O2.
Statistical Analysis
Data were statistically analyzed using mean ± standard deviation. Mann-Whitney test (U) was used to analyze nonparametric continuous variables, or small groups. Independent samples t-test with Bonferroni correction was used to compare continuous variables with normal distribution. We used Pearson correlation coefficient (two-tailed) for correlation between parameters. P value of <0.05 was considered statistically significant. Microsoft Excel software and the Statistical Package for the Social Sciences software (SPSS Inc., Chicago, IL) were used for calculations.
Result
There was no significant history for pre/peri-natal data for both groups. Psychomotor development-data taken from parents showed a normal development according to age stages. Neurological examination was normal in both groups. Clinical evaluation showed no clinical deficits or history of vision and hearing deficits. Clinical examination showed no signs of psychopathology pediatric field. Psychological testing using Raven’s progressive matrices showed an IQ above 85 in all children (between 87 and 120). In neither group were children with global intellectual deficits. Were obtained relative powers for frequency bands delta (0.5-3.5Hz), theta (4.0- 7.5Hz), alpha (8.0-12.5Hz) and beta (13.0-30.0Hz) at the electrodes: FP1, FP2, F7, F8, C3, C4, T5, T6, P3, P4, O1, O2.
Figure 1:The representation of relative power for each frequency band according to age group set for EEG maturation: 3-6 years, 6-12 years, 13-19 years.
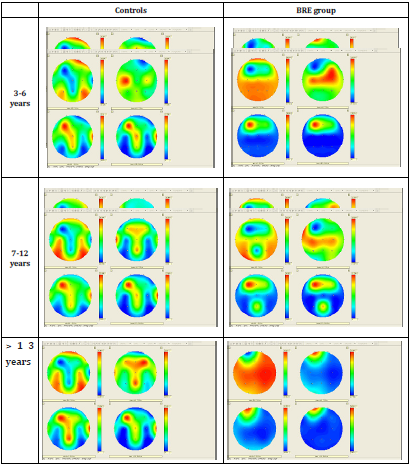
It was a dominance of delta relative power in both groups, 73.58-77.79% in the BRE, and 68.11-78.37% in the control group, maximum representation at BRE group occipital, and occipital, central and frontal in control group. Followed, the relative power of theta frequency band: 16.93-19.6% -BRE group and 14.88-22.99%-control group, and the relative power of alpha frequency band: 4.1-4.77%-BRE group and 5.28-7.13% in control group, both in the fronto-central maximum. Relative power of beta frequency band was between 1.15-2.28% in the BRE and 1.13- 2.43% in the control group, both with a frontal maximum. We mention a more uniform distribution of relative power for each frequency band the BRE group compared with the control group.
In (Figure 1) are represented the relative powers of the frequency of BRE and control group. We compared medium of relative powers between BRE and control group. We found no significant differences in theta and beta frequency bands; regarding delta frequency band-there were significant differences at C3 electrode, where delta relative power is higher in the BRE patients (t=2.12, p<0.05) and relative power of alpha frequencywas lower at the same C3 electrod, in BRE group compared with the control group (t=2.39, p<0.05). Comparison between left and right hemisphere of the relative powers of all frequency bands showed some left/right differences. Note that both groups were righthanded. In the control group-delta relative power dominate in the right hemisphere fronto-central: Fp1/Fp2 (71.73/78.37, t=2.74, p<0.05), F7/F8 (71.89/74.92, t=2.64, p<0.05), C3/C4 (68.11/71.76, t=3.70, p<0.01), relative theta power-dominant in left hemisphere fronto-central: Fp1/Fp2 (19.36/14.88, t=3.00, p<0.01), F7 / F8 (19.95/17.85, t=2.79, p<0.05), C3/C4 (22.99/20.75, t=3.33, p<0.01), alpha relative power-dominant on left hemisphere, fronto-central: Fp1/Fp2 (6.46/5.02, t=2.43, p<0.05), C3/C4 (7.13/5.97, t=2.60, p<0.05) and beta relative power-dominant in left hemisphere, in the central and occipital regions: C3/C4 (1.75/1.51, t=2.73, p<0.05) and O1/O2 (1.34/1.13, t=3.04, p<0.01). In BRE group-there were no significant differences in left/right relative powers of delta, theta and alpha. For beta relative power, there is a dominance at the level of electrodes C3/C4 (1.42/1.55, t=2.32, p<0.05).
We computed the delta/theta ratio, evaluated at each electrode, compared between BRE and control group. There were no differences in the ratio delta/theta, except for electrode FP2, but even here, the difference is at the limit of statistical significance (t=2.01, p=0.05). the theta/delta ratio, comparison (at the 12 electrodes) between the two groups showed significant differences at C3 electrode (t=2.33, p<0.05). the theta/beta ratio showed no significant differences for any of the electrodes. Amplitudes in each electrode were evaluated and compared between the control and BRE group-for sequences provided quantitative processing program, namely: 9.131sec, 9.622sec, 10.132sec and 10.623sec. For sequence 9131sec-there were significant differences at the electrode T5 (t=2.05, p<0.05), also were significant differences at C3 electrode (t=2.04, p<0.05) for sequence 10,132sec. There were no significant differences for sequences 9.622sec, respectively, 10.623sec, for any of the electrodes.b
Discussion
AAN (American Association of Neurology), defines QEEG as “Mathematical processing of digital EEG, to highlight specific parts of the waves, and converts a digital EEG route in a format that can provide relevant information” [17,18]. Data can be stored for future comparisons. EEG power-the sum of neurons discharges synchronously, it depends on several factors: thickness scalp, CSF, distance between electrodes, age [17]. Visual analysis of the sleep EEG background revealed no pathological changes. Delta power was dominant (70%), more expressed at occipital (BRE) and fronto-central (control), without differences statistically significant between groups. A dominant power in NREM sleep higher than 16Hz, is correlated with a state of hypervigilance and increased brain activity, as shown QEEG studies reported in people with primary insomnia [16].
NREM sleep is better represented in children compared with adolescents, between the hours 1-4 of sleep [19]. Slow waves have a maximum dominance occipital in preschoolers, parietal and frontal regions back to school-age children (8-14 years), in adolescents (over 14 years) there is a dominance of slow waves at the frontal regions. Slow waves ratio fronto/occipital is independent of age but remain steady throughout the night [20]. The results suggest an “immaturity” of BRE group compared with the control group. There are changes of sleep structure during childhood, mostly related to the aging process; decrease of slow-wave sleep, increase of stage 2 sleep, associated with reduce of stages 3 and 4 and reduce the total sleep time. From 6 to 15 years of age, NREM sleep is characterized by a progressive decrease of delta waves, especially in stages 3 and 4. These changes were related to hormonal changes and ontogenetic alterations of cortical synaptic density (that reaches a peak in the first decade of life), then suffer a major reorganization in the second decade [19]. It was hypothesized that slow sleep reflects synaptic activity; when learning process during the day is intense, involving more neural networks for cortical synaptic potentiation, the more expressed is the activity in slow sleep [20].
There were significant differences between BRE and control group in the left central region (C3), where dominant frequencies were from delta ranges (t=2.12, p<0.05) at the expense of high frequencies-alpha (t=2.39, p<0.05) in the BRE group compared with the control group. This finding suggests the presence of brain dysfunction in the central regions. Slow activity is the manifestation of a brain disorder, best correlate with the presence of structural lesions [21,22]. Data from BRE group suggest rather a functional alteration, possibly related to the maturation of the central region. Benchmarking amplitude between BRE and control group, showed significant differences in the central regions (C3) and temporal (T5), suggesting an involvement, at least functional of the region. We mention that longitudinal assemblies were used, which makes the validity of these differences smaller in terms of amplitude. Amplitude evaluation is done on referential montages, as the amplitude is largely dependent on the distance between the electrodes [21]. There were evaluated patients with symptomatic focal epilepsy frontal and temporal, and it was found a marked reduction in alpha activity and a moderate increase in theta power in all patients, regardless of epilepsy syndrome variant. These deviations from the normal distribution had a wide cortex distribution and were not related with anti-epileptics.
Focal epileptogenesis has a widespread impact on EEG frequency composition [23]. Corroborating our results with these studies, we suggest rather a delay of brain maturation in these children than the presence of structural damage. Further studies on larger population are needed, however to confirm this hypothesis. In children, there is reported an increased absolute theta power throughout the night, with a decline in the first two hours of sleep. It is considered that there is a direct relationship between the decline of NREM sleep and decrease theta power. It is reported a higher alpha power in children compared with adults throughout NREM sleep, [19,24]. [25] have identified a theta and delta absolute power higher in children with BRE compared with control group; and negative correlations between theta power at the different electrodes and intellectual development of these children. These results were related with maturation disorders of brain electrical activity and tend to lower cognitive performance [25,16].
Interhemispheric left/right comparison of relative power for each frequency band showed greater variability in the control group versus the BRE group. There are studies showing significant differences in the rate of maturation for the left and right hemispheric [26]. All assessed children were righthanded, the more likely that these differences to be related to brain maturation, showing physiological differences between right and left hemisphere-in the control group, and a more ‚uniform, cortical activity-in BRE group. Differences in the beta frequency band, with central dominance are likely related to sleep spindles (both having the same frequency range). Data proved that there are significantly more theta activity and less alpha activity in girls than in boys, under the age of 6 years; but considering the developmental rhythm is evaluated to be higher and lasts longer in boys than girls, girls will get boys later the development spurt [26]. Given the small group of patients and controls in the study, and lack of sex concordance between groups, we could not assess whether there are differences related to the sex of children.
Evaluation of delta/theta, theta/delta and theta/beta ratios revealed no significant differences between BRE and control group, except ratio theta/delta which was lower in the BRE group at the C3 electrode (t=2.33, p<0.05). It suggests differences in low frequency bands in the central regions in children with BRE, possibly caused by a brain dysfunction in this region. Theta/delta shift in BRE children during sleep, in the central regions, is not present. Theta/alpha ratio it is best correlated with age and is independent of derivations evaluated. Before the age of 8 years, alpha frequency is dominant in central regions compared with the occipital ones, later this dominance is reversed [26]. There are studies that report a relation between theta/beta ratio and cognitive impairment (present in early stages of Alzheimer dementia), while in physiological aging is reported a theta/alpha ratio increased [27]. Theta/beta ratio in awake EEG examinations-is deemed to be non-specific for the diagnosis of children with ADHD. This ratio is higher in children with ADHD compared to the control group, where there is an increased theta activity in combination with decreasing alpha activity [28]. Theta/beta ratio was not altered in BRE group compared with the control group, these results suggest that attention disorders, or ADHD-like symptoms that may be present in these children appear through involvement of other brain circuits.
Study Limitations
This study has some limitations, resulting from the difficulties present in most clinical trials that include pediatric age patient populations with relatively rare disease, compared with the general population. Number of children enrolled was small but typical for studies on groups of children with epilepsy. Noted, however, that they were included in the study only children at seizures onset, before starting the AED therapy.
Conclusion
Our results have shown electroencephalographic alterations of sleep background at the level of epileptic focus and differences possibly related to a brain immaturity in BRE children. In BRE children there is an excess of slow waves (delta) and amplitude differences compared with healthy children in NREM sleep, in Centro-temporal regions, possibly related to a functional alteration of the brain regions. Delta power was dominant (70%), more expressed at occipital (BRE) and fronto-central (control), without differences statistically significant between groups. Delta relative power is higher in the BRE patients and relative power of alpha frequency-was lower at the level of central areas (C3). In BRE group, there were no significant differences in relative powers of delta, theta and alpha between left/right hemisphere; while in control group we found a greater variability, with lower frequencies (delta) were dominant on the right hemisphere and higher frequencies (theta, alpha) were dominant on the left hemisphere, probably related to impaired maturation of brain electrical activity in NREM sleep in these children. Analyzing delta/theta, theta/delta and theta/beta ratios revealed no significant differences, except for theta/delta ratio, which was lower in the BRE group at the central region (C3 electrode), reflecting the epileptic focus.
References
- Amrutkar C, Rosario M, Romero R (2018) Rolandic epilepsy (BRE) seizure. StatPearls [Internet].
- Kubota M, Diep T, Hirose H, Kimura I, Sakakihara Y (2004) Patients with benign rolandic epilepsy have longer duration of somatosensory evoked high frequency oscillation. Pediatr Int 46(6): 631-634.
- Ferrie CD (2008) Benign focal epilepsies in childhood. In Pellock BB (Ed.), JM Pediatric Epilepsy. Diagnosis and Therapy (3rd edn), DEMOS, New York, USA, pp. 31-45.
- Ishitobi M, Nakasota N, Yamamoto K, Iinuma K (2005) Opercular to interhemispheric source of distribution of benign rolandic spikes of childhood. Neuroimage 25(2): 417-423.
- Gerrie CD (2008) Benign focal epilepsies of childhood. În Pellock BB (Ed.), JM Pediatric Epilepsy. Diagnosis and Therapy (3rd edn), DEMOS, New York, USA, pp. 335-350.
- Lundberg S (2004) Rolandic epilepsy a neuroradiological, neuropsychological and oromotor study. University dissertation from Uppsala: Acta Universitatis Upsaliensis, Sweden 1332: 1-80
- Vischer CV, Ingvar MM, Picard F, Mayordubois C, Davidoff V (2002) Epileptic falls and gait disturbances in two young children with a sharp wave focus at the vertex: a variant of benign partial epilepsy of childhood? European Journal of Pediatric Neurology 6(3): 169-178.
- Winawer MR, Shin J, Beck ES, Hunter JE, Investigators E, et al (2016) Genetic effects on sleep/wake variation of seizures. Epilepsia 57(4): 557-565.
- Thatcher RW (2005) EEG and intelligence: relations between EEG coherence, EEG phase delay and power. Clin Neurophysiol 116(9):2129-2141.
- Medeiros Kanda de PA, Renato Anghinah, Magali Taino Smidth, Silva JM (2009) The clinical use of quantitative EEG in cognitive disorders. Dement Neuropsychol 3(3):195-203.
- Binnie CD (2003) Clinical Neuropsychology. In EEG, Paediatric Neuropsychology, Special Techniques and Applications Elsevier Science BV, pp. 131-150.
- https://emedicine.medscape.com/article/1140635-overview
- Özge A, Toros F, Cömelekoğlu U (2004) The role of hemispheral asymmetry and regional activity of quantitative EEG in children with stuttering. Child Psychiatry Hum Dev 34(4): 269-280.
- Hallioglu O, Ozge A, Comelekoglu U, Topaloglu AK, Kanik A, et al (2001) Evaluation of cerebral maturation by visual and quantitative analysis of resting electroencephalography in children with primary nocturnal enuresis. J Child Neurol 16(10): 714-718.
- https://www.pediatrics.emory.edu/divisions/neurology/education/pedeeg.html
- Skrijelj F, Sokić D (2011) Dilemmas in diagnostics and therapy of rolandic epilepsy. Vojnosanit Pregl 68(6): 526-528.
- Wendorff J, Przygocka J, Juchniewicz B (2006) Cognitive disturbances in rolandic epilepsy-correlation with electroencephalographic patterns. Przegl Lek 63(1):14-17.
- Nuwer M (1997) Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American academy of neurology and the American clinical neurophysiology society. Neurology 49(1): 277-292.
- Johnson Markve BL, Lee GP, Loring DW, Viner KM (2011) Usefulness of verbal selective reminding in distinguishing frontal lobe memory disorders in epilepsy. Epilepsy Behav 22(2): 313-317.
- Striano PZF (2011) Genetic epilepsies. European Journal of Paediatric Neurology 15: 88-89.
- Danielsson J, Petermann F (2009) Cognitive deficits in children with benign rolandic epilepsy of childhood or rolandic discharges: a study of children between 4 and 7 years of age with and without seizures compared with healthy controls. Epilepsy Behav 16(4): 646-651.
- Tovia E, Goldberg Stern H, Ben Zeev B, Heyman E, Watemberg N, et al (2011) The prevalence of atypical presentation and comorbidities of benign childhood epilepsy with centrotemporal spikes. Epilepsia 52(8): 1483-1488.
- Coben LA, Danziger WL, Berg L (1983) Frequency analysis of the resting awake EEG in mild senile dementia of Alzheimer type. Electroencephalogr Clin Neurophysiol 55(4): 372-380.
- Merica H, Fortune RD (2005) Spectral power time-courses of human sleep EEG reveal a striking discontinuity at approximately 18Hz marking the division between NREM-specific and wake/REM-specific fast frequency activity. Cereb Cortex 15(7): 877-884.
- Tedrus Gloria MA, (2006) Benign childhood epilepsy with centro-temporal spikes: quantitative EEG and the wechsler intelligence scale for children (WISC-Ill). Clinical Eeg and Neuroscience 37(3): 193-197.
- Genizi J, Shamay Tsoory SG, Shahar E, Yaniv S, Aharon Perez J (2012) Impaired social behavior in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol 27(2): 156-161.
- Bate LGM (1999) Genetics of inherited epilepsy. Epileptic Disorders pp. 1-7.
- Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, et al (2008) EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep 31(12): 1673-1682.
© 2019 Vintan Mihaela Adela. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






