- Submissions

Full Text
Significances of Bioengineering & Biosciences
Nanomaterial Toxicity and Regulatory Framework: Need of the Hour
Satbir Singh1*, Komal Dagar2, Kehar Singh1, Divya Kaushik2 and Baburam3
1Associate Professor, School of Pharmacy, Lingaya’s Vidyapeeth, India
2Assistant Professor, Pt. LR College of Pharmacy, India
3Associate Professor, Jeevan Jyoti Pharmacy and Medical Science, India
*Corresponding author:Satbir Singh, Associate Professor, School of Pharmacy, Lingaya’s Vidyapeeth, Haryana, India
Submission: June 17, 2025; Published: August 04, 2025

ISSN 2637-8078Volume7 Issue4
Abstract
Nanoparticles (NPs) and Nano-Structured Materials (NSMs) represent a dynamic area of research and a fast-expanding techno-economic sector in diverse applications. Nano-materials exhibit multiple traits that make them suitable for diverse clinical applications. Their defining characteristics include super para-magnetism, high agglomeration and mechanical properties, along with a significant surface area-to-volume ratio. Monometallic nano-structured metal clusters act as precursors for heterogeneous catalysts. Nano Pharmaceuticals (NPs) are characterized as nano-materials that have at least one external dimension, or who’s internal or surface structure is within the nanoscale range (about 1 to 100nm). NPs have distinct physical and chemical properties that affect their toxicity, in contrast to those of larger counterparts. Research into nano-toxicity centers on the detrimental impacts of nano-materials, with the goal of determining their potential threat level to society and the environment. The FDA utilizes a product-focused and science-based regulatory policy that leverages this emerging technology to regulate products. Guidelines cover its physicochemical properties, biological properties and product applications.
Graphical Abstract
Figure 1:Graphical abstract for nanomaterial toxicity and regulatory framework.
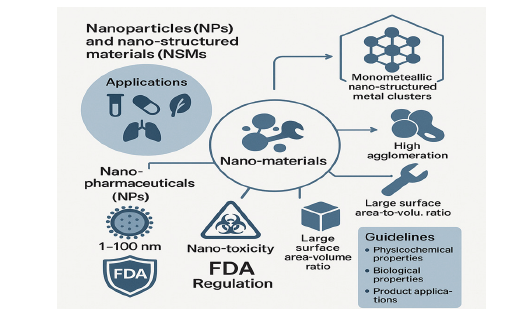
Keywords: Nanoparticles; Toxicity; Regulations; FDA; EMEA; Nanomaterials
Introduction
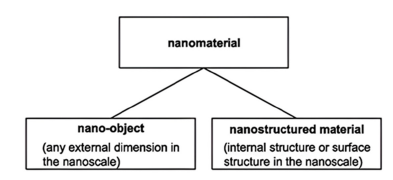
A nanomaterial is characterized as a “material with any external dimension within the nanoscale or possessing internal structure or surface structure at the nanoscale,” where nanoscale is defined as “the length range approximately from 1nm to 100nm.” This encompasses both nano-objects, which are individual pieces of material and nano-structured materials, which possess internal or surface structures at the nano scale; it is possible for a nano material to belong to both of these categories [1]. Nanoparticles (NPs) and Nano-Structured Materials (NSMs) constitute a burgeoning field of study and a developing technoeconomic sector across various applications [2]. NPs and NSMs have become prominent in technological advancements because of their tunable physicochemical properties, such as melting point, wettability, electrical and thermal conduction and scattering (Figure 2). These characteristics lead to improved performance compared to their bulk counterparts [3]. Various countries having their different opinion regarding nanomaterials which are defined as follows:
Figure 2:Types of nanomaterials [2].
According to European Commission cosmetics directive (EC 2009)
Nanomaterial is defined as follows: ‘Nanomaterial’ refers to an intentionally produced substance that is insoluble or biopersistent, with one or more external dimensions or an internal structure at a scale of 1 to 100nm. As per the definition provided by the European Commission, a nanomaterial is a natural, incidental or manufactured substance that contains particles-either in an unbound state, as aggregates or as agglomerates-and where 50% or more of the particles in its number size distribution have one or more external dimensions ranging from 1nm to 100nm [4].
According to Australian government
Nanomaterial refers to industrial materials that are deliberately produced, manufactured or designed to possess unique characteristics or a specific composition at the nanoscale, which typically ranges from 1nm to 100nm. These materials can be classified as either nano-objects (confined at the nanoscale in one, two or three dimensions) or nanostructured (having structures at the nanoscale on its surface or internally). Aggregates and agglomerates are encompassed and pertain to materials in which 10% or more of the particles, based on number count, fulfill the aforementioned definition [5].
According to Canada
Health Canada defines nanomaterial as any manufactured substance or product, including its component materials, ingredients, devices or structures, that meets one of the following criteria: It has at least one external dimension at the nanoscale; its internal or surface structure is at the nanoscale; it is not at the nanoscale in all dimensions but demonstrates one or more properties or phenomena characteristic of the nanoscale. Nanoscale pertains to characteristics that can be ascribed to the dimensions of the material and size effects [6].
According to USA (United States food and drug administration)
A formal definition of agency does not exist. However, when assessing if an FDA-regulated product contains nanomaterials or involves nanotechnology, the FDA will inquire: Does the engineered material or final product have at least one dimension within the nanoscale range (approximately 1nm to 100nm)? or whether a manufactured material or final product displays characteristics or occurrences, such as physical or chemical properties or biological effects, that can be linked to its dimension(s), even if these dimensions exceed the nanoscale range but are within one micrometer [5,6].
Types and Classification of Nanomaterials
Most current NPs and NSMs can be categorized into four material-based groups: Nanostructured material characterized by a microstructure with a characteristic length scale of a few (typically 1-10) nanometers and NPs, referred to as Nanostructured material [7,8].
Carbon-based nanomaterials
These NMs typically consist of carbon and can be found in forms like hollow tubes, ellipsoids or spheres. Fullerenes (C60), Carbon Nanotubes (CNTs), carbon nano fibers, carbon black, Graphene (Gr) and carbon onions fall under the category of carbon-based NMs. These carbon-based materials (except carbon black) are primarily produced using laser ablation, arc discharge and Chemical Vapor Deposition (CVD) [7].
Inorganic-based nanomaterials
These NMs comprise metal and metal oxide NPs as well as NSMs. NMs like these can be synthesized into various forms, including metals such as Au or Ag NPs, metal oxides like TiO2 and ZnO NPs, as well as semiconductors such as silicon and ceramics.
Organic-based nanomaterials
This comprises NMs that are primarily composed of organic matter, while excluding carbon-based or inorganic-based NMs. By employing non-covalent (weak) interactions for self-assembly and molecular design, organic NMs can be converted into target structures like dendrimers, micelles, liposomes and polymer NPs.
Composite-based nanomaterials
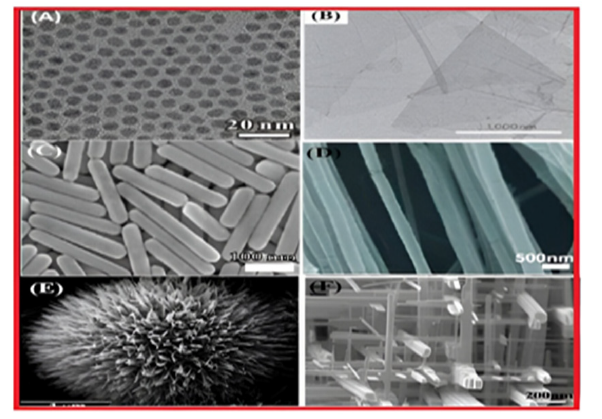
Composite NMs are multiphase NPs and NSMs where one phase is at the nanoscale. They can consist of combinations of NPs with other NPs, or with larger or bulk materials (e.g., hybrid nanofibers), as well as more complex structures like metal-organic frameworks. The composites can consist of any combinations of carbon-based, metal-based or organic-based NMs with bulk materials made from metal, ceramic or polymer. NMs are produced in various morphologies, as indicated in Figure 3, based on the necessary characteristics for the intended application.
Figure 3:Various types of morphologies of nanomaterials [7].
Classification Based on Origins
Apart from dimension and material-based classifications, NPs
and NSMs can also be classified as natural or synthetic, based on
their origin.
A. Natural nanomaterials are produced in nature either by
biological species. The production of artificial surfaces with
exclusive micro and nanoscale templates and properties for
technological applications are readily available from natural
sources [9].
B. Synthetic (engineered) nanomaterials are produced
by mechanical grinding, engine exhaust and smoke or are
synthesized by physical, chemical, biological or hybrid methods
[10].
Classification of Nanomaterials on the Basis of Dimension
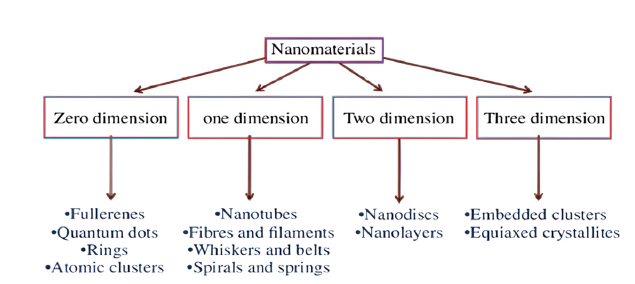
The Gleiter scheme categorizes nanomaterials based on crystalline forms and chemical compositions. However, it was incomplete due to not considering dimensionality. In Pokropivny et al. [11], developed a new classification scheme for nanomaterials, including 0D, 1D, 2D and 3D composites, reflecting electron movement along dimensions [11].
The small nanomaterials have various dimensions like:
a) Zero dimensions.
b) One dimension.
c) Two dimensions.
d) Three dimensions.
The dimensions of nanomaterials and some of their important characteristics are explained in Figure 4. The nanomaterials have at least one dimension of 1-100nm. The nanomaterials exist in single, aggregated or fused forms with several shapes such as tubular, spherical and irregular. The most common types of nanomaterials are nanofibers, nanotubes, quantum dots and nanosheets. Siegel also classified nanomaterials into four types: zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructures [11,12].
Figure 4:Dimensions of nanomaterials and their characteristics.
Properties of Nanomaterials
Nanomaterials have several properties that make them suitable
for a variety of clinical applications.
A. One of the major benefits of nanoparticles is their small
size of 10-200nm allowing them to circulate the body without
disrupting blood flow, as well as being able to avoid clearance
by both the renal and complement systems [13].
B. Size of nanomaterials is relevant in treatment of cancer
because of enhanced permeability and retention effect was one
of the ways that nanoparticles could successfully penetrate
tumour tissues. Reference must be in ascending order [14].
C. Nanomedicines are generally simple and cheap to
manufacture on the small scale, however, difficulty with scale
up and stability on large scale manufacture has been widely
experienced [15,16].
Sources of nanomaterial
Sources of nanomaterials can be classified into below main
categories based on their origin:
a) Incidental nanomaterials: These are generated as
byproducts of industrial processes, including nanoparticles
from vehicle engine exhaust, welding fumes, combustion
processes and even natural events like forest fires [17].
b) Nanomaterials may be incidentally produced as a
byproduct of mechanical or industrial processes: Incidental
nanoparticles can originate from vehicle engine exhaust,
welding fumes and combustion processes involved in domestic
solid fuel heating and cooking. As an example, fullerenes-a
category of nanomaterials-are produced by the combustion of
gas, biomass and candles [18].
Additionally, it may result from wear and corrosion products (Figure 5). Incidental atmospheric nanoparticles, known as ultrafine particles, are unintentionally generated during deliberate activities and may contribute to air pollution [19,20].
Figure 5:Sources of nanomaterials [19].
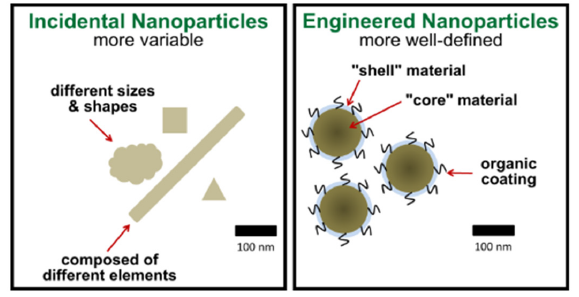
Nanoparticles
Engineered nanomaterials
Human-designed nanomaterials, including carbon black and titanium dioxide nanoparticles, are incremental improvements over colloidal or particulate materials, with specific properties for their intended uses, prior to nanotechnology [21].
Naturally produced nanomaterials
Nanomaterials are found in various organisms, including insects, plants, animals and humans. Natural organic nanomaterials include foraminifera, viruses, wax crystals, silk and more. Inorganic nanomaterials form through crystal growth under earth’s crust, such as clays and opals. Insectal nanomaterials may be a subcategory of natural nanomaterials. Natural inorganic nanomaterials, like clays and opals, form due to complex chemical reactions in fires. Natural sources of nanoparticles comprise combustion products from forest fires, volcanic ash, ocean spray and the radioactive decay of radon gas. Natural nanomaterials can form through the weathering of rocks containing metals or anions and also at locations impacted by acid mine drainage [22].
Advantages of nanomaterials
The nanomaterials’ electrical, magnetic, optical and mechanical
properties have led to numerous intriguing applications. Research
is ongoing to learn about these properties. The nanomaterials
exhibit properties that differ from those of bulk-sized models. The
following are some benefits of the nanomaterials:
A. At high temperatures, nanophase ceramics exhibit greater
ductility than coarse-grained ceramics.
B. The nanosized metallic powders exhibit a high coldwelding
property and ductility, making them highly useful for
metal-metal bonding.
C. Solo magnetic particles at the nanoscale exhibit super
paramagnetic properties.
D. Nanostructured metal clusters made up of a single metal
serve as precursors for heterogeneous catalysts.
E. Nanocrystalline silicon films create a contact that is highly
transparent for solar cells.
F. Porous films of nanostructured titanium oxide provide
high transmission and enhancement due to their high surface
area.
G. The microelectronic industry’s challenges in circuit
miniaturization, including inadequate heat dissipation
from high-speed microprocessors and low reliability, can
be addressed with nanocrystalline materials. These offer
excellent thermal conductivity, great durability and long-lasting
interconnections.
Disadvantages
Some of disadvantages are as follows:
a) Instability of then a no materials.
b) Poor corrosion resistance.
c) High solubility.
d) When the nanomaterials with the high surface area come
in direct contact with oxygen exothermic combustion takes
place leading to an explosion.
e) Impurity.
f) Nanomaterials are considered to be biologically harmful.
These have high toxicity which can lead to irritations.
g) Carcinogenic.
h) Difficult to synthesize.
i) No safe disposal available [23].
Toxicology of nanomaterials
Nanotoxicology examines the harmful effects of nanomaterials, focusing on their unique characteristics and potential dangers. Inhalation exposure, skin contact and ingestion are the most concerning. It evaluates the toxicological characteristics of nanoparticles to determine their environmental and societal threats. Nanomaterials have various applications but can also be toxic [24].
Properties that affect toxicity
Size is a key factor in assessing particle toxicity, but other factors like chemical composition, morphology, surface architecture, surface charge, aggregation state, solubility and functional groups also affect toxicity, making generalizations challenging [25].
Composition
Metal-based
Metal-based Nanoparticles (NPs) are used in biomedicine for drug delivery systems, but recent advancements in nanotechnology have led to studies evaluating their environmental impact. Researchers have found that certain metal and metal oxide nanoparticles can cause DNA damage, mutations, reduced cell viability, apoptosis, necrosis and reduced proliferation [26].
Carbon-based
As of 2013, the most recent toxicology studies on mice involving Carbon Nanotube (CNT) exposure indicated that MWCNT has a limited pulmonary inflammatory potential at levels that correspond to the average inhalable elemental carbon concentrations found in CNT facilities in the US. According to the study, considerable years of exposure are required for significant pathology to develop [27].
Other
Other classes of nanomaterials include polymers such as nanocellulose and dendrimers.
Size
The size of a nanoparticle can influence its toxicity in various ways. As an illustration, lung deposition and clearance rates vary for particles of differing sizes. The particles’ reactivity and the exact mechanism of their toxicity can also be influenced by size [28].
Dispersion state
Agglomeration or aggregate of nanoparticles in environmental or biological fluids is a common phenomenon, with ISO and ASTM defining it differently. Agglomeration affects nanotoxicity due to its impact on size, surface area and sedimentation characteristics. Mechanical stability of airborne engineered nanoparticle clusters also influences size distribution. Different aerosolization and deagglomeration systems test nanoparticle agglomerates’ stability [29].
Surface chemistry and charge
In their application, nanomaterials are coated and can be assigned positive or negative charges based on their intended purpose. Research has shown that these external elements influence how toxic nanomaterials can be [30].
Toxicity of Nanomaterials Found in Human Environment
Respiratory
Nanoparticles in the workplace are primarily inhaled, with their size and shape influencing their deposition in the lungs. Animal studies suggest they can enter the bloodstream and migrate to other organs, including the brain. The risk of inhalation is influenced by the dustiness of the material and its likelihood of being lifted into the air. Carbon nanotubes and carbon nanofibers can cause pulmonary effects like inflammation, granulomas and pulmonary fibrosis. Pulmonary exposure can lead to genotoxic or carcinogenic effects, as well as systemic cardiovascular effects. As of 2013, there were no reported adverse health effects in workers using or producing these nanomaterials [31].
Dermal
Studies suggest that nanomaterials can penetrate the body through unbroken skin during occupational exposure. Particles with diameters less than 1μm can infiltrate mechanically flexed skin and pigs’ skin. Factors like dimensions, morphology, water solubility and surface treatment affect nanoparticle penetration. Current research shows dermal irritation in mice and pro-inflammatory cytokines in human skin cells [32].
Gastrointestinal
Materials can be ingested through inadvertent transfer from hand to mouth. This has been observed with conventional materials and it is a scientifically valid assumption that it could also occur when handling nanomaterials. Particles that are removed from the respiratory tract by the mucociliary escalator can be ingested, which means that ingestion may occur alongside inhalation exposure [33].
Bio-distribution
Nanomaterials, due to their small size, can easily penetrate the human body, but their behavior depends on size, shape and surface reaction. Overwhelming phagocytes could cause inflammation and reduce resistance to pathogens. Nanoparticles can also disrupt biological processes, as they adsorb macromolecules upon contact with tissues and fluids. They can enter the bloodstream through inhalation or ingestion and can be absorbed by various organs and tissues. The composition and concentration of nanomaterials can determine their toxicity, leading to oxidative stress, inflammatory cytokines and cell death [34].
Regulations Framework of Different Countries
Regulatory framework in India
A nano pharmaceutical is characterized as a pharmaceutical formulation that includes nanomaterials and is designed for internal or external use on the body to provide therapeutic effects, diagnostic purposes or any health benefits. It contains materials measuring between 1 and 100nm in at least one dimension. Furthermore, if the particle size exceeds 100nm, it will still fall within the definition as long as it possesses altered or different pharmaceutical characteristics in relation to the application of nanotechnology compared to those of the API [35]. The “Guidelines for Evaluation of Nanopharmaceuticals in India” categorizes nanopharmaceuticals based on biodegradability, nature, ingredient nanoforms and approval status. It also provides information on toxicological and clinical trial data. The guidelines apply to new drug approvals and clinical trials. Due to the complexities of nanotechnology, a specialized approach is required. The ministry of health regulates nanotechnology applications through its directorate general of health services, overseeing the Central Drugs Standard Control Organization (CDSCO) [36].
Regulatory framework in USA
The FDA has faced numerous risks and uncertainties linked
to emerging technologies and nanotechnology is no exception.
FDA monitors the fruiting science with robust research agenda in
order to help assess the safety and effectiveness of products using
nanotechnology [37]. The FDA uses a product-focused regulatory
policy based on science and emerging technology, utilizing legal
standards for different product categories. It plans to oversee
nanotechnology products using current legal authorities, based on
specific criteria. The FDA has not established regulatory definitions
for terms like “nanotechnology,” “nanomaterial” or “nanoscale”
which refer to the engineering of materials with dimensions
between 1 and 100 nanometers. Nanotechnology is defined by the
National Nanotechnology Initiative Program [34]. To check if FDAregulated
product involves the application of nanotechnology, FDA
will ask:
A. Whether a material or final product is designed to have at
least one external dimension or an internal or surface structure,
within the nanoscale range (approximately 1nm to 100nm).
B. Whether a material or final product is designed to
display characteristics or phenomena-such as physical or
chemical properties or biological effects-that can be linked to
its dimensions, even if these dimensions are not within the
nanoscale range but extend up to one micrometer (1,000nm)
[36].
The USFDA has developed guidance focused on human drug
products, including biological products, that contain nanomaterials
in their finished dosage form. This guidance addresses Critical
Quality Attributes (CQAs), manufacturing processes, stability, Good
Manufacturing Practices (GMP), post-marketing controls, clinical
and non-clinical developments and potential risks related to their
use [19]. The USFDA recommends a risk-based strategy for drug
product development containing nanomaterials, emphasizing risk
factors. The CQA examines nanomaterials’ chemical composition,
particle size, distribution, shape and stability. Standardized
methods are being developed for nanomaterial characterization.
Manufacturing process and in-process controls must consider
nanomaterials in the manufacturing process. For manufacturing
process and in-process controls, products containing nanomaterials
must be manufactured with reference to:
a) cGMP in section 501(a)(2)(B) of FD&C Act.
b) cGMP regulations in CFR parts 210, 211 and 212.
The clinical development of drug products containing nanomaterials shall follow all policies and guidance with respect to clinical safety and efficacy studies pertaining to the development of IND, NDA, ANDA and BLA submissions [38].
United States
A. The ASTM committee manages the coordination of ASTM
standardization and encompasses guidance and standards for
nanomaterials in nanotechnology.
B. USFDA oversees a diverse array of products, including
drugs, food, cosmetics, etc., that employ nanotechnology
[39]. For all the products, they have guidelines. The size of
nanomaterials can influence their physicochemical properties.
The properties of the same component may differ for their size.
Guidelines are provided for its physicochemical properties,
biological characteristics and the use of those products.
The FDA activities are:
a) The guidance provided by CDER for nanoparticle
development encompasses risk and quality assessments of
nanoproducts, along with standards for their identification,
physicochemical properties, evaluation and use.
b) To create a regulation that can adapt to changing
circumstances.
c) To promote collaboration and alliances with stakeholders.
d) To enhance the knowledge base of regulatory science.
e) To create a product-centric and scientifically grounded
method for governing nanomaterials [40,41].
The US Environmental Protection Agency (EPA) was established
in 2017 to oversee the health and safety of nanoscale materials,
aiming to regulate them as a single class and incorporate this into
risk-based principles. A rule was issued in January 2017 mandating
one-time reporting and recordkeeping of exposure and health
information for nano-sized chemicals [42,43]. This includes,
A. Production volume.
B. Manufacturing method.
C. Processing, uses, exposure and release information.
D. Health and safety data.
Regulatory framework in Europe
Several EU legislative and technical guidance documents have been drafted by the European Commission, containing precise references to nanomaterials [44]. The term ‘nanomaterial’ refers to a natural, incidental or manufactured material that contains particles-either in an unbound state, as aggregates or as agglomerates-and for which 50% or more of the particles in the number size distribution have one or more external dimensions within the range of 1nm to 100nm. Fullerenes, graphene flakes and single-wall carbon nanotubes with one or more external dimensions below 1nm shall be classified as nanomaterials, as defined in the reference [45].
ECHA (European Chemical Agency)
ECHA collaborates with member state competent authorities, the European Commission, stakeholders and international organizations such as the Organization for Economic Cooperation and Development (OECD) [46]. REACH and CLP encompass nanomaterials, involving tasks in various REACH processes (such as registration, evaluation, authorization and restrictions) and CLP processes (including Classification and Labeling) for nanoforms [47].
China
China’s CFDA governs nanomaterial regulations, including nano pharmaceutical production, approval and inspection processes for drugs and medical devices. The CFDA is divided into two areas: Registration and tracking. Medical devices are classified into Class I, Class II and Class III, with Class I being straightforward and Class III being intricate implants. Drugs are categorized into chemical, biological and traditional Chinese medicine or natural drugs according to China’s pharmaceutical classification system [48].
South Korea
South Korea’s drug and medical device approval process is overseen by the Ministry of Food and Drug Safety (MFDS), which also manages pharmaceutical registration and distribution. The Ministry of Health and Welfare (MOHW) makes decisions on coverage, coding and pricing. The Medical Device Act (MDA) governs current regulations, classifying medical devices into four categories based on risk: Class I, Class II, Class III and Class IV [49].
Brazil
ANVISA, the National Health Surveillance Agency, was set up as a financially autonomous entity in Brazil on January 26, 1999. It is associated with the Ministry of Health and overseen by a board of directors composed of multiple members. This agency is tasked with approving food, pharmaceuticals, health services, medical devices and more. ANVISA serves as the overarching authority for medical devices technology, overseeing the guidelines related to medical devices during their development and in the context of drug approval [50,51]. The manufacturer can start their application process before obtaining the GMP certificate and with this, Brazil began the reconstruction and enhancement of its regulatory system.
United Kingdom
For all pharmaceutical companies using nanomaterials, the MHRA oversees the legislation and regulations. It has established standard operating procedures regarding safety, quality and performance [52]. All novel drugs that are newly created receive legislative approval under the MHRA for “first-in-man” studies and review information is recorded through post-market surveillance. Procedures that have received less attention are not governed by any legislative regulations [53-55].
Conclusion
To sum up, despite the promising applications of nanomaterials in diverse areas, their distinctive characteristics can lead to serious health hazards-especially via inhalation and skin contact-which makes it essential to establish strict regulations to guarantee safe usage. To completely comprehend their toxicological effects and to create efficient protective strategies for workers and consumers, it is essential to conduct ongoing research and thorough evaluations.
Acknowledgement
Author expresses his sincere thanks to all authors for their valuable contribution to carry out this review work.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- ISO/TS 80004-1: 2015 (2018) Nanotechnologies-vocabulary-part1: Core terms. International Organization for Standardization.
- ISO/TS 80008-1 (2010) Nanotechnologies-vocabulary-part1: Core terms.
- Gaffet E (2011) Nanomaterials: A review of the definitions, applications, health effects. How to implement secure development. Nanomaterials Research Group, Belfort Cedex, France.
- ACC (2011) Comments submitted in response to: Pesticides; policies concerning products containing nanomaterials; opportunity for public comment (EP10-0197). American Chemistry Council, Washington, USA.
- FDA (2014) Guidance for industry considering whether an FDA-regulated product involves the application of nanotechnology, p. 3.
- Health Canada (2014) Policy statement on health Canada’s working definition for nanomaterial.
- Kumar N, Kumbhat S (2016) Carbon-based nanomaterials. Essentials in Nanoscience and Nanotechnology pp. 189-236.
- Gleiter H (2000) Nanostructured materials: basic concepts and microstructure. Acta Materialia 48(1): 1-29.
- Hochella MF, Spencer MG, Jones KL (2015) Nanotechnology: Nature's gift or scientists' brainchild? Environ Sci Nano 2(2): 114-119.
- Wagner S, Gondikas A, Neubauer E, Hofmann T, Kammer F (2014) Spot the difference: Engineered and natural nanoparticles in the environment-release, behavior and fate. Angew Chem Int Ed Engl 53(46): 12398-12419.
- Singh V, Yadav P, Mishra V (2020) Recent advances on classification, properties, synthesis and characterization of nanomaterials. Green Synthesis of Nanomaterials for Bioenergy Applications, pp. 83-97.
- Pokropivny VV, Skorokhod VV (2007) Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater Sci Eng: C 27(5-8): 990-993.
- Satbir SS (2017) Global regulatory challenges of common technical document. World Journal of Pharmacy and Pharmaceutical Sciences 6(12): 1298-1309.
- Golombek SK, May JN, Theek B, Appold L, Drude N, et al. (2018) Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Delivery Rev 130: 17-38.
- Paliwal R, Babu RJ, Palakurthi S (2014) Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 15(6): 1527-1534.
- Rachel Foulkes, Ernest Man, Jasmine Thind, Suet Yeung, Abigail Joy, et al. (2020) The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci 8(17): 4653-4664.
- Taylor DA (2002) Dust in the wind. Environ Health Perspect 110(2): A80-A87.
- Barcelo D, Farre M (2012) Analysis and risk of nanomaterials in environmental and food samples. Elsevier, Oxford, p. 291.
- Portela CM, Vidyasagar A, Krödel S, Weissenbach T, Yee DW, et al. (2020) Extreme mechanical resilience of self-assembled nanolabyrinthine materials. Proc Natl Acad Sci USA 117(11): 5686-5693.
- Hoover M, Myers D, Cash LJ, Guilmette R, Kreyling W (2017) Radiation safety aspects of nanotechnology. National Council on Radiation Protection and Measurements, Bethesda, Maryland, USA, pp. 88-90.
- (2017) A new integrated approach for risk assessment and management of nanotechnologies. EUSustainable Nanotechnologies Project, pp. 109-112.
- Satbir S, Harsh M, Kehar S, Hemant R (2025) A review on chemistry, synthesis, extraction, nano-formulation and therapeutic use of curcumin. Asian Journal of Research in Pharmaceutical Sciences 15(2): 135-140.
- Buzea C, Pacheco I, Robbie K (2007) Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2(4): MR17-71.
- Oberdorster E, Zhu SQ, Blickley TM, Clellan GP, Haasch ML (2006) Ecotoxicology of carbon-based engineered nanoparticles: Effects of fullerene (C60) on aquatic organisms. Carbon 44(6): 1112-1120.
- Kehar S, Satbir S, Shiv K, Vikram (2024) A review on nanoparticles and targeted drug delivery system for cancer therapy. International Journal of Research Publication and Reviews 5(5): 12417-12422.
- Cassano D, Pocoví MS, Voliani V (2018) Ultrasmall-in-nano approach: Enabling the translation of metal nanomaterials to clinics. Bioconjugate Chemistry 29(1): 4-16.
- Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761): 622-627.
- Seabra AB, Durán N (2015) Nanotoxicology of metal oxide nanoparticles. Metals 5(2): 934-975.
- Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, et al. (2010) Metal-based nano particles and their toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2(5): 544-568.
- Cassano D, Santi M, Cappello, Luin S, Signore G, et al. (2016) Biodegradable passion fruit-like nano-architectures as carriers for cisplatin prodrug. Particle & Particle Systems Characterization 33(11): 818-824.
- Erdely A, Dahm M, Chen BT, Zeidler E, Fernback JE, et al. (2013) Carbon nanotube dosimetry: From workplace exposure assessment to inhalation toxicology. Particle and Fibre Toxicology 10(1): 53.
- Martins IJ (2018) Increased risk for obesity and diabetes with neurodegeneration in developing countries. In Top 10 Contributions on Genetics, pp. 1-35.
- Martins I (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research 5(1): 9-26.
- (2009) Approaches to safe nanotechnology: Managing the health and safety concerns associated with engineered nanomaterials. National Institute for Occupational Safety and Health, Washington, USA, pp.11-12.
- (2012) General safe practices for working with engineered nanomaterials in research laboratories. National Institute for Occupational Safety and Health, Washington, USA, pp. 5-6.
- (2013) Current intelligence bulletin 65: Occupational exposure to carbon nanotubes and nanofibers. National Institute for Occupational Safety and Health, Washington, USA, pp. 63-64.
- (2011) Current intelligence bulletin 63: Occupational exposure to titanium dioxide. National Institute for Occupational Safety and Health, Washington, USA, pp. 73-78.
- Holsapple MP, Farland WH, Landry TD, Monteiro RN, Carter JM, et al. (2005) Research strategies for safety evaluation of nanomaterials part II: Toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicological Sciences 88(1): 12-17.
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, et al. (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Particle and Fibre Toxicology 2: 8.
- Hoet PH, Brüske HI, Salata OV (2004) Nanoparticles-known and unknown health risks. Journal of Nano-biotechnology 2(1): 12.
- Satbir S, Kehar S, Komal D, Divya K, Baburam (2025) A review on role of nanotechnology in plant disease management. World Journal Medical & Pharmaceutical Research 1(2): 1-9.
- Paliwal R, Babu RJ, Palakurthi S (2014) Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 15(6): 1527-1534.
- Prepared reflection paper on nanotechnology-based medicinal products for human use. Eur Med Agency Guidelines for Evaluation of Nanopharmaceuticals in India.
- Guidelines and best practices for safe handling of nanomaterials in research laboratories and industries. Nano Mission, DST, Govt of India.
- Park HG, Yeo MK (2016) Nanomaterial regulatory policy for human health and environment. Molecular & Cellular Toxicology 12: 223-236.
- FDA’s approach to regulation of nanotechnology products. FDA.
- Nanotechnology fact sheet. FDA.
- (2014) Considering whether an FDA-regulated product involves the application of nano-technology.
- FDA (2017) Drug products, including biological products that contain nanomaterials guidance for industry. FDA.
- Flinders Consulting Pty (2006) A review of the potential occupational health and safety implications of nano-technology.
- (2014) National nanotechnology initiative strategic plan. National Science and Technology Council Subcommittee on Nanoscale Science, Engineering and Technology.
- (2013) Nanotechnology regulatory science research plan.
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J Nanotechnol 9: 1050-1074.
- Nanomaterials-ECHA.
- Campbell B (2013) Regulation and safe adoption of new medical devices and procedures. Br Med Bull 107(1):5-18.
© 2025 Satbir Singh*, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






