- Submissions

Full Text
Significances of Bioengineering & Biosciences
Utilization of Mahogany Seed Oil as a Renewable Feedstock for Cleaner Biodiesel Production
Biyas Ghosh1 and Manager Rajdeo Singh2*
1National Research laboratory for Conservation of Cultural property, Mysore Centre, Mysore, India
2Department of Tourism Administration, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India
*Corresponding author:Manager Rajdeo Singh, Department of Tourism Administration, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India
Submission: October 17, 2024; Published: October 25, 2024

ISSN 2637-8078Volume7 Issue1
Abstract
This study examines the potential of Mahogany Seed Oil (MSO) as a non-edible feedstock for biodiesel production, providing an eco-friendly substitute for petroleum-based fuels. The FFA content of the oil was reduced to zero using acid-catalyzed esterification, followed by base-catalyzed transesterification to convert the esterified product into monoesters and glycerol. Various parameters such as solvent and catalyst quantity, temperature and reaction time were optimized to enhance conversion efficiency. The properties of MSO and MSO biodiesel, including heating value, were compared to those of commercial diesel. LC-MS analysis confirmed the conversion of MSO to biodiesel, and the oxidative stability of MSO biodiesel was evaluated. Key oil characteristics, including saponification, iodine, FFA, peroxide and acid values, fell within acceptable ranges compared to another biodiesel feedstocks. The study concludes that MSO represents a promising source for biodiesel production, offering an alternative to conventional diesel fuel. This research provides valuable insights into the potential utilization of non-edible feedstock’s like Swietenia mahogany in future transportation fuel applications.
Keywords:Swietenia mahogany; Soxhlet extraction; Transesterification; Free fatty acid; Antioxidant; Biodiesel
Abbreviations:PM: Particulate Matter; MSO: Mahogany Seed Oil; EV: Electric Vehicles; HEV: Hybrid Electric Vehicles; PHEV: Plug in Electric Vehicles; BEV: Battery Electric Vehicle; FCEV: Fuel Cell Electric Vehicles; S. MAHOGANY: Swietenia Mahogany; FFA: Free Fatty Acid; FAME: Fatty Acid Methyl Ester; SVO: Straight Vegetable Oil; LC-MS: Liquid Chromatography-Mass Spectrometry
Introduction
The depletion and growing scarcity of traditional fossil fuels, coupled with escalating petroleum prices and environmental apprehensions, have spurred a heightened interest in alternative energy sources. Among these, biodiesel has emerged as a promising option for transportation fuel. Its popularity has soared due to its potential to serve as a sustainable substitute for conventional diesel, offering significant emission reduction benefits [1].
Air pollution is a pressing public health concern, and the energy sector plays a significant role in both contributing to and mitigating this issue. Since the early 2000s, efforts have been underway to implement strategies for reducing emissions [2]. Despite the anticipated increase in fuel combustion, projections indicate a reduction in Particulate Matter (PM) emissions by 7%, sulfur dioxide by 20% and nitrogen oxides by 10% by 2040 [3], aiming to meet the rising global energy demand [4]. In urban areas, motor vehicles are the primary source of fine particulate matter, particularly PM2.5, which poses health risks upon exposure. These minute particles have been linked to adverse health effects in humans.
Utilizing non-edible sources for biodiesel production offers a promising environmentally friendly alternative to petroleum fuel [5]. The process involves reducing the Free Fatty Acid (FFA) content of the oil to zero through acid-catalyzed esterification, followed by base-catalyzed transesterification. Biodiesel production relies on synthetic reactions such as transesterification and esterification to produce biofuel. Typically, short-chain alcohols react with vegetable or animal fats, with ethanol being a popular choice due to its ease of use. Although methanol may result in higher biodiesel conversions, basecatalyzed transesterification is commonly preferred due to its shorter reaction times and lower cost compared to acid catalysis. However, base catalysis is sensitive to water and free unsaturated fats in oils.
The potential substitute
Oils and fats have long been considered as viable alternatives to diesel fuel [6]. These natural resources are readily available and their glycerides can effectively replace diesel fuel. Various countries select specific oil sources as substitutes for diesel fuel, depending on their climate and soil conditions [7]. For example, soybean oil is commonly utilized for biodiesel production in Southeast Asia, particularly in Malaysia and Indonesia [8]. In contrast, the Philippines predominantly use coconut oil for this purpose.
Biodiesel is increasingly recognized as a non-toxic, biodegradable and renewable alternative to traditional diesel fuel [9]. Essentially, biodiesel comprises monoalkyl esters derived from renewable sources and its potential for compression-ignition engines has garnered interest from numerous commercial fuel producers. Researchers have been investigating biodiesel and its properties to understand its remarkable similarities to diesel, especially in light of the limited supply of petroleum-based fuels [10].
The utilization of vegetable oil in diesel engines is not a recent concept. However, its significance has grown as concerns over the depletion of petroleum fuels and the need for environmentally sustainable alternatives have become more apparent. Numerous studies have explored alternatives to biodiesel production from various seed oils [11]. Key parameters required to assess the quality of biodiesel include viscosity, cloud point, pour point, flash point, Higher Heating Value (HHV) and cetane number. Typically, these properties are higher before the transesterification process. However, after conversion, properties like viscosity, cloud point and flash point significantly decrease. Viscosity, in particular, plays a critical role as it can impact automobile emissions. Higher viscosity at lower temperatures may affect the fuel injection system, leading to reduced atomization and erratic fuel burning. The cloud point of a fuel signifies the temperature at which it solidifies, impacting its viscosity at lower temperatures. A lower cloud point implies a lesser likelihood of higher viscosity at low temperatures. On the other hand, the flash point denotes the temperature at which it ignites. Fuels with higher flash points require more energy to ignite, leading to increased emissions. Therefore, monitoring these characteristics is crucial as they influence the concentrations of fuel combustion emissions.
Benefits of biodiesel
Biodiesel provides various environmental advantages, with its primary benefit being its designation as “carbon neutral.” This means that the fuel’s combustion does not result in a net release of carbon dioxide (CO2) emissions. The growth of oilseed crops absorbs a comparable amount of CO2 as is released during fuel combustion, effectively offsetting its carbon footprint. However, it’s crucial to acknowledge that CO2 emissions also occur during the production of compost used in cultivating oil crops, which raises complexities regarding the carbon neutrality claim. When it comes to spillage, biodiesel poses less harm compared to fossil fuels and exhibits higher flammability than fossil diesel. This characteristic enhances safety in the event of accidents.
Basic biodiesel production
The three main methods for producing biodiesel are:
A. Transesterification: The most commonly employed and
efficient method for producing biodiesel is the transesterification
process. It entails reacting oil or fat with an alcohol using a base
catalyst. This method operates at moderate temperatures and
pressures, typically yielding around 98 percent.
B. Acid-catalyzed transesterification: It involves the reaction
between oil or fat and an alcohol in the presence of an acid catalyst.
However, it is not as commonly employed as base-catalyzed
transesterification due to lower conversion yields and more
challenging reaction conditions.
C. Oil to fatty acids conversion: In this method, the oil is
first converted into fatty acids, which are then transformed into
biodiesel [12]. This process involves additional steps compared
to transesterification methods and is less commonly used in
biodiesel production. Overall, base-catalyzed transesterification
is the desired method due to its efficiency, relatively mild reaction
conditions and high conversion yields.
Transesterification is a chemical process involving the reaction of a triglyceride (fat/oil) with an alcohol. Triglycerides are composed of unsaturated fats bonded to a glycerol molecule. The composition of unsaturated fats influences the properties of the fat, which, in turn, affects the quality of the biodiesel produced from it. During esterification, the triglyceride reacts with alcohol in the presence of a catalyst, typically a solid base like sodium hydroxide. This reaction with unsaturated lipids forms mono-alkyl esters, known as biodiesel and crude glycerol as a by-product. Methanol or ethanol is commonly employed as the alcohol, with the reaction catalyzed by potassium or sodium hydroxide. Methanol yields methyl esters, while ethanol produces ethyl esters. Although potassium hydroxide is preferred for ethyl ester biodiesel production, either base can be used for methyl ester production. Rape Methyl Ester (RME), derived from crude rapeseed oil reacting with methanol, is a typical product of the transesterification process. The schematic representation of the methyl ester biodiesel method is illustrated in Figure 1. Since the reaction between fat or oil and alcohol is reversible, a large quantity of alcohol must be added to drive the reaction forward and ensure complete conversion.
Figure 1:Reaction method for biodiesel and glycerol production.

Glycerol, as the denser by-product, settles and can be marketed directly or refined for diverse industrial applications, such as pharmaceuticals and cosmetics. Straight Vegetable Oil (SVO) serves various purposes yet substituting it for fossil diesel may pose several engine challenges. The high viscosity of SVO can impede fuel atomization, leading to incomplete combustion, injector blockages and piston ring corrosion. Furthermore, carbonization and fuel buildup may affect lubricating oil performance. To mitigate these issues, the transesterification of the oil is advised.
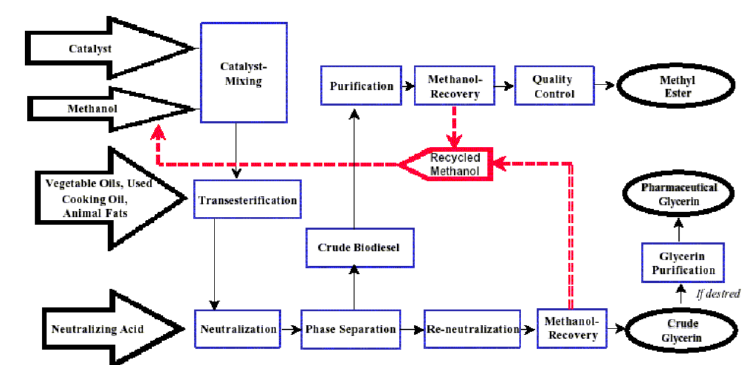
Below is a simplified production flow chart Figure 2
accompanied by a concise description of each step:
Before being utilized as commercial fuel, the finalized biodiesel
undergoes rigorous testing using advanced technology to ensure it
meets all specified requirements.
Figure 2:Production flow chart of biodiesel.
Review of Literature
In recent years, there has been a significant increase in both the production and consumption of biofuels, surpassing initial expectations. However, studies have shown that first-generation biofuels have limited capacity to replace fossil fuels and mitigate Greenhouse Gas (GHG) emissions. This realization has underscored the urgent need for a transition to second-generation biofuels. Compared to their first-generation counterparts, these biofuels are expected to have lower agricultural land requirements, leading to more favorable energy balances, greater reductions in GHG emissions and reduced competition for crop land with food crops.
As traditional petroleum-based gasoline reserves dwindle and environmental concerns intensify, biofuel emerges as a promising alternative fuel option. However, complete substitution with biofuel is currently hindered by limitations in production capacity and compatibility issues with existing engines. Nonetheless, utilizing biofuel as a substitute for diesel could help mitigate the impact of vehicular pollution on climate patterns [13]. The global push for biofuel development is driven by concerns regarding energy security and climate change, both of which have the potential to stimulate growth in the agricultural industry. However, challenges persist, including long-term compatibility of vehicle engines and concerns about food safety arising from the production of biofuel from food-grade oilseeds. Biodiesel usage can also result in significant corrosion, carbon buildup and wear of engine components, as well as the degradation of fuel delivery system components [14]. After carefully weighing the advantages and disadvantages of biodiesel, it becomes evident that a dedicated biodiesel engine is the optimal choice for its commercialization [15]. Brazil successfully promoted bioethanol adoption by introducing Flexible-Fuel Vehicles (FFVs), which can run on both ethanol and gasoline. A similar approach could facilitate the widespread adoption of biofuels. Therefore, the development of specialized biofuel engines is crucial for the extensive commercialization and utilization of biofuels.
The annual energy derived from biomass generated by terrestrial plants is estimated to be 3-4 times greater than the current global energy consumption. Solid biofuels, including firewood, wood chips, wood pellets and hardwood charcoal, are commonly utilized examples. While the worldwide consumption of firewood and charcoal has remained relatively consistent, the use of wood chips and pellets for electricity generation (biopower) and domestic heating has quadrupled over the past decade and is anticipated to continue rising in the future.
Liquid biofuels, which encompass bioethanol, biodiesel, pyrolysis bio-oil and drop-in transportation fuels, represent another category. The commercial production of bioethanol from lignocellulosic materials has recently commenced, supplementing the annual supply of 22 billion gallons primarily derived from agricultural crops. Flammable biofuels, including biogas and syngas, also play a significant role. In Europe and China, there has been notable growth in the production of biogas from organic waste through anaerobic digestion. The evolution and global utilization of bioenergy and biofuels, particularly in sectors such as biopower, lignocellulosic bioethanol and biogas, are expected to continue [16]. It is projected that by 2050, bioenergy will contribute to meeting 30% of the world’s electricity needs [17].
From a commercial standpoint, conventional petroleum companies may perceive non-ester renewable diesel fuels as more familiar and thus more comfortable than biodiesel. This could potentially impede the widespread adoption of biodiesel as an alternative fuel in the future. However, despite this obstacle, there are signs indicating that the nascent biodiesel industry will garner robust government backing.
Numerous nations are currently enhancing the biodiesel industry through fiscal and tax policies, alongside establishing national standards for manufacturing methods, product quality and manufacturing safety to standardize production processes. Concurrently, governments must effectively address unresolved issues concerning biofuel, such as food safety, land use changes and forest conservation [18]. Liquid fuels derived from renewable biomass could play a crucial role in reducing fossil fuel consumption and consequently, mitigating global warming. Nonetheless, several challenges must be tackled to realize this vision on a large scale, including the environmental impact of biomass harvesting or cultivation, ensuring adequate water and energy supply to expanded biomass operations and establishing and scaling up commercial systems for extracting and refining biofuels at competitive costs.
Scientists worldwide are diligently working to develop biofuel feedstocks that can efficiently convert food crops into energy, particularly as the world grapples with food shortages and escalating fuel prices. While many oils suitable for human consumption may be the most affordable feedstock for biofuel production, it’s evident that the demand for biodiesel is projected to increase in the near future. However, meeting this growing demand sustainably poses a significant challenge. This underscores the need to explore non-edible oilseeds as a reliable, long-term feedstock for biofuel production [19]. Additionally, most non-edible seeds have the potential to reclaim wilderness areas and do not compete with food crops for limited arable land. Therefore, identifying suitable non-edible feedstocks and assessing their viability for biodiesel production will be crucial [20].
Butanol is a significant microbial product with the potential to transition from renewable carbohydrate sources to a “drop-in” liquid biofuel, thereby expanding its market scope. The promising performance of butanol as a straight or mixed fuel in specific engines has sparked extensive research into revitalizing the ancient Acetone-Butanol-Ethanol (ABE) fermentation process from both technological and environmental perspectives [21].
Vegetable oils are naturally occurring in various plants, each offering its own unique oil. With the increasing demand for vegetable oils in recent years, the oil-processing industry has been exploring new exploitable resources, including the recycling of agricultural waste. Various techniques, including cold pressing, traditional solid-liquid extractions such as maceration and Soxhlet extractions and non-conventional extractions like microwave, ultrasound and supercritical fluid extractions using solvents such as hexane and petroleum ether, have been utilized for the production of edible oils. These methods are accompanied by the analysis of physicochemical qualities such as refractive index, color perception, melting point, viscosity, iodine value, saponification value and peroxide value to ensure the quality and suitability of the oils for various applications [22].
Second-generation biofuels offer a more sustainable approach to fuel production by utilizing plants that are not suitable for consumption, thus avoiding direct competition with food production [23]. The objective of this approach is to achieve a product with a neutral or low net carbon footprint, which can be accomplished using agricultural waste, straw, grass and wood. Further research is necessary to ensure the stability of raw material supply and its utilization in biodiesel production. Microbial oil-based biodiesel holds promise for completely meeting fuel demand, but challenges exist in producing bacteria, particularly due to their temperature sensitivity and inability to thrive in hot climates. To address the energy crisis through biodiesel usage, waste oils and non-edible oils should be incorporated into the biodiesel production process. New, cost-effective oil extraction methods such as supercritical and microwave-assisted transesterification are recommended for oil extraction [24].
Second-generation biofuels offer a more sustainable approach to fuel production by utilizing plants that are not suitable for consumption, thus avoiding direct competition with food production [23]. The objective of this approach is to achieve a product with a neutral or low net carbon footprint, which can be accomplished using agricultural waste, straw, grass and wood. Further research is necessary to ensure the stability of raw material supply and its utilization in biodiesel production. Microbial oil-based biodiesel holds promise for completely meeting fuel demand, but challenges exist in producing bacteria, particularly due to their temperature sensitivity and inability to thrive in hot climates. To address the energy crisis through biodiesel usage, waste oils and non-edible oils should be incorporated into the biodiesel production process. New, cost-effective oil extraction methods such as supercritical and microwave-assisted transesterification are recommended for oil extraction [9].
Production of Biodiesel from Non-Edible Sources
Biomass energy is derived from naturally growing plants, many of which produce seeds with high oil content suitable for use as biofuel. India, with its rich biodiversity, boasts over 150 varieties of oil-producing plants that could be utilized industrially for energy production [25]. The growing global awareness of biofuels stems from their potential to enhance energy security, create employment opportunities and generate higher income for rural communities.
The benefits of non-edible plants in biofuel production include their ability to be cultivated along the edges of farmlands, homes, streets and barren terrains where access to land resources is available [26]. This decentralized approach allows each household to grow non-edible oilseed plants in their homestead areas, along boundaries, courtyards and accessible areas, providing a convenient and accessible source of raw materials for biofuel production.
Biofuels, being renewable resources, offer several advantages over fossil fuels. Ethanol, for example, is considered the most cost-effective fuel worldwide according to the Renewable Fuels Association’s February 2019 Ethanol Industry Outlook study. Unlike fossil fuels, biofuels emit fewer greenhouse gases, resulting in reduced environmental impact and helping to combat global warming. Moreover, as crude oil becomes scarcer in certain regions, biofuels offer a viable alternative and reduce the government’s reliance on fossil fuels. Additionally, some biofuels require oilproducing plants or crops, providing farmers with an opportunity to generate additional income [27]. The expansion of biofuel companies can also create more job opportunities, contributing to economic stability.
Biofuels offer several environmental benefits due to their biodegradability, resulting in reduced land and subsurface water contamination during transit, storage and consumption. These qualities contribute to biodiesel’s flexibility and appeal in the current energy landscape, enhancing energy flow, climatic sustainability and rural uplift by transitioning from petroleum to agricultural sources [28].
At present, several countries in Asia, including Indonesia, Malaysia, the Philippines, Thailand, China and India, are leading producers of biofuels. Among these, Malaysia and Indonesia stand out as top palm oil producers, driving palm biodiesel production in Southeast Asia due to its high potential and yield factor [29]. According to data from the Ministry of Energy and Mineral Resources, Indonesia produced 520,000 tons of biodiesel in 2007. Both Thailand and Indonesia have consistently advanced in biofuel production, benefiting from the availability of diverse feedstock.
According to the International Energy Agency’s World Energy Outlook 2007 study, global energy consumption is expected to rise by 50% by 2030, with China and India driving 45% of this growth [30]. In India, coal serves as the primary energy source for power generation, while petroleum fuels transportation [31]. The Ministry of Petroleum and Natural Gas launched the first phase of Ethanol Blended Petrol (EBP) in 2003, blending 5% ethanol into gasoline across nine states and four union territories. The national Mission on Biodiesel, initiated in April 2003, has identified Jatropha as the most suitable oilseed plant for biodiesel production [32].
India’s diverse agro-climatic conditions support the successful cultivation of Tree-Borne Oilseeds (TBOs) [33]. Nevertheless, the expansion of oilseed cultivation faces limitations due to escalating land demand resulting from urbanization and industrialization. TBOs possess significant oil potential and could play a substantial role in achieving self-sufficiency in vegetable oil production in the near future.
Tree-borne oilseeds such as Mahua (Madhuca Indica), Neem (Azadirachta Indica), Simarouba (Simarouba Glauca), Karanja (Pongamia Pinnata), Ratanjyot (Jatropha Circus), Jojoba, Kokum (Garcinia Indica), Wild Apricot (Prunus Armeniaca), Wild Walnut, and others native to India can be cultivated and established in wasteland and various other areas [34,35]. These oilseeds have both household and industrial applications but remain underutilized due to factors such as limited understanding of their applications, insufficient processing facilities and an unorganized marketing sector [36].
Tree Borne Oil Seeds
a) Calophyllum inophyllum (Sura honne): The Guttiferae/ Clusiaceae plants are commonly found in coastal regions and hilly areas of peninsular India. These plants produce seed kernels that yield approximately 50-73% of dark green viscous oil. This oil serves various purposes including soap making, burning and as biodiesel. Additionally, when mixed with resin from Vateria indica, it is utilized for caulking boats along the West Coast.
b) Madhuca butyracea (Diploknema butyracea): Sapotaceae, commonly referred to as the “Indian Butter tree,” produces oil commercially known as “Phulwara Butter,” which shares a consistency similar to ghee. This oil is utilized for various purposes including the adulteration of ghee, in soap and candle production and as an ingredient in chocolate manufacture. Additionally, the oil cake derived from this tree is used as a form of organic manure.
c) Garcinia indica: Guttiferae/Clusiaceae, specifically Garcinia Indica, commonly known as Kokam, is renowned for its rich antioxidant properties. The edible fat derived from Kokam, known as “Kokam Butter,” is frequently utilized as an adulterant for ghee. Additionally, the cake residue from Kokam processing serves as valuable organic manure. Indigenous to the Western Ghats region of India, Kokam is a significant botanical resource in the area.
d) Hydnocarpus kurzii (Chaulmugra oil): Flacourtiaceae comprises evergreen shrubs within the Achariaceae family, typically reaching heights of 20-30 meters and widths of 15 meters. These shrubs are predominantly found in East Asia. The kernels of these plants yield oil ranging from 16% to 36%, which is utilized in the treatment of leprosy and various skin diseases. Additionally, the residue left after oil extraction, known as seed cake, serves as an effective fertilizer.
e) Hydnocarpus laurifolia: The kernels of Flacourtiaceae plants contain approximately 63% oil, which is utilized in the treatment of leprosy, wounds, ulcers and various skin diseases. The residue left after oil extraction, known as seed cake, is valued as a nutrient-rich manure for agricultural purposes.
f) Madhuca indica (Mahua): The seed oil from Flacourtiaceae, also known as “Mahua butter,” “Iluppai butter,” or “Bassia butter,” serves various purposes. It is utilized in soap production, cooking, hair oil, medicinal applications, and as an adulterant for ghee.
g) Mallotus philippensis (Kamela/ Kumkuma): Euphorbiaceae plants yield oil suitable as a substitute for Tung oil, commonly used in quick-drying paints and varnishes. This oil is also incorporated into the formulation of hair fixers and ointments. Additionally, the residue from oil extraction, known as cake, is valued as an organic fertilizer.
h) Melia azedarach (Persian Lilac): Meliaceae, also known as the chinaberry tree, pride of India, bead-tree, Cape lilac, syringa berry tree, Persian lilac, Indian lilac or white cedar, belongs to the mahogany family, Meliaceae and is native to the Indomalaya region. It is often grown as an ornamental tree and is deciduous in nature. The seeds of this tree yield a drying oil, constituting about 40% of the seed content, which is renowned for its delightful fragrance. This oil finds applications in the manufacturing of soaps and hair oils.
i) Mesua ferrea/ M. Nagassarium (Naga sampige): Guttiferae/Clusiaceae oil, characterized by a brown hue and a mildly unpleasant scent, serves various purposes including burning, lubrication and soap making. However, caution is advised as the cake residue contains poisonous acid resin, acting as a heart poison. Therefore, it is not suitable for use as cattle feed. Nevertheless, it is valued as a nutrient-rich manure due to its high nitrogen and phosphorus content.
j) Mimusops elengi (Bullet wood/ Bakula/Ranjala): Sapotaceae, this medium-sized evergreen tree is prevalent in tropical forests across South Asia and Southeast Asia. Often cultivated along avenues, it serves as both an ornamental and shade tree. The seed kernels yield fatty oil, comprising 16-25% of their content, which is utilized for both edible and lighting purposes.
k) Pongamia pinnata (Karanj): Fabaceae plants produce seeds that yield a yellowish-brown oil, valued in medicine as an antiseptic and for soap making. Additionally, this oil finds application in the leather industry and is considered excellent for biodiesel production. The residue left after oil extraction, known as oil cake, can be utilized as a nutrient-rich manure for paddy cultivation
l) Schleichera oleosa: (Ceylon oak, Kusmi, Macassar oil tree, Sagadi): Sapindaceae seeds produce Kusum oil, a yellowish-brown oil used for cooking, lighting and soap making. It is also known as the Original Macassar oil, prized for its properties in cleaning and promoting hair growth.
m) Vateria indica: (Vellapine, Indian Copal tree, White dammar, Dhupada mara): Dipterocarpaceae seeds yield a solid oil known as “Piney tallow,” utilized for lighting, as a ghee substitute, in candle production, and for medicinal purposes. This oil is also employed in confectionery and as a dressing for chronic rheumatism.
n) Jatropha curcus: Jatropha curcas, a versatile oilseed tree or shrub, thrives in subtropical and tropical regions worldwide. Its oil possesses a high cetane rating and low sulfur content, making it highly desirable for biodiesel production. In India, it is regarded as the most politically and morally acceptable biofuel option, with no known negative impact on the production of essential agricultural goods such as grains.
o) Melia dubia: Melia dubia, commonly known as Malabar Neem, is widely cultivated not only in Karnataka but also in various parts of South India. This species thrives in a range of habitats including moist deciduous, evergreen and semi-evergreen forests.
p) Pterocarpus marsupium: Scientific Name: Pterocarpus marsupium Roxb. Family: Fabaceae Subfamily: Faboideae, Vernacular/Common Names: Kannada: Honne, English: Kino tree, Hindi: Bijasal.
Seed Treatment
The modified versions for preparing Pterocarpus marsupium seeds for sowing is as under: Cutting the ends of the pods and soaking them in water for a few days before sowing. Creating alternate layers of pods and leaves in a pit, flooding it with water until germination begins, then sowing the germinated pods in the nursery. Soaking the pods in a slurry of cow dung, camphor water or plain cold water. Occasionally, enclosing pods in a cloth or gunny bag and soaking them in water for 24 hours before sowing. For this study specific tree borne oil yielding seed named Swietenia Mahogany, currently available in the different regions of district Uttara Kannada of Karnataka state, South India was selected [5,37].
Case Study
Swietenia Mahogany: Swietenia Mahogany is a substantial semi-evergreen tree, often reaching heights of up to 75 feet and spreading out to around 50 feet. However, it is commonly observed at heights of 40 to 50 feet with a similar spread. The robust, durable wood of the Mahogany tree is highly resistant to wind damage, making it an ideal choice for planting a shade tree or along streets. Swietenia wood is used for the extraction of oil by pyrolysis process and maximum yield is around 70% [38-40].
A. Taxonomy and nomenclature: Swietenia Mahogany, also
known as Swietenia belizensis Lundell, Swietenia candollei Pittier
and by various other synonyms, belongs to the Meliaceae family of
plants. Within this family, it falls under the subfamily Swietenoideae.
In Indonesia, it is commonly referred to as “Mahoni,” a vernacular
name used throughout the country. In Bangladesh, it is known as
“Bara mahauni” or “bara-mahagoni,” while in other countries,
it is simply referred to as “mahogany” or “big- or large-leaved
mahogany” [41].
B. Field characteristics: This tree is a large deciduous
species with an umbrella-shaped crown, often reaching heights
of over 30 meters and boasting a diameter exceeding 1.5 meters
at breast height. Its trunk is straight and cylindrical, adorned with
well-defined spurs and a slight groove. Younger trees typically
have a narrow crown, while mature specimens display a broad,
thick and densely branching crown. The crown itself is open and
rounded, featuring thick, ascending branches and dense foliage.
The outer bark of older trees is scaly, shaggy, heavily longitudinally
wrinkled and ranges in color from brownish-grey to reddishbrown,
with inner bark varying from red-brown to pinkish-red.
Leaves are typically paripinnate, occasionally imparipinnate and
measure between 12 to 45 centimeters in length, with 3 to 6 pairs
of lanceolate or ovate leaflets.
C. Flowers: The flowers of this species exhibit hues of white
or golden. While not particularly showy, they emit a pleasant aroma
and cluster together in axillary panicles. These fragrant blooms
typically emerge during the spring season.
D. Fruit: The fruit of this species is oval-shaped, typically
measuring between 2 to 5 inches in length. When mature, the fruit
covering becomes dry or hard, enclosing a woody capsule that
splits into five even sections. The color of the fruit is brown. These
fruits lack appeal to wildlife, are easily visible and can pose a litter
issue. Fruiting occurs from summer to winter.
E. Trunk and Branches: The trunk and branches of this
species typically droop and are not particularly striking. Typically,
there is a single trunk, and the tree does not bear thorns. As the
tree matures, the bark transitions from gray to dark gray, becoming
tough, scaly and flaking off to reveal red areas. Pruning is essential
to establish a strong framework. The tree exhibits greater resistance
to breakage than to bending.
F. Environment: species thrives in full sun to moderate
shade conditions. It shows tolerance to various soil types including
clay, sand and loam, and can adapt to both acidic and alkaline soil
pH levels. It prefers soil that is damp but well-drained. Additionally,
it exhibits strong drought tolerance and is highly tolerant to salt in
aerosols.
G. Distribution: This species has been widely planted across
Southern Asia and the Pacific, particularly in countries like India,
Indonesia, the Philippines and Sri Lanka. It has also been introduced
to West Africa. In its native range, which includes Belize, Bolivia,
Brazil, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala,
Honduras, Mexico, Nicaragua, Panama, Peru and Venezuela, the
species is widespread [42]. However, in certain regions such as
Ecuador, Colombia, Panama and Costa Rica, it faces the threat of
extinction. There are concerns about commercial extinction in
Bolivia and its population is decreasing in Mexico, Belize and Brazil.
H. Seed collection: This species is typically propagated
from seeds, with the best results achieved by collecting seeds
from a healthy mother tree. Seed production can vary significantly
from year to year. The fruits are ideally collected from the ground
immediately after seed fall or from the trees themselves just before
they open. This can be done either by climbing the trees or by using
poles with metal hooks to cut down the seeds.
I. Seed preparation: Mature dry fruits or seeds (capsules)
gathered from the forest floor can be stored for several days in
sacks without notable deterioration. However, unripe capsules
may require initial drying to aid in their opening. This can be
accomplished by sun-drying the capsules or placing them on a rack
over electric lamps at a temperature of 38 °C for 36-48 hours to
encourage them to open.
J. Applications: Due to its high-quality wood, this species
is well-suited for large-scale wood manufacturing plantations.
The wood is commonly used in the production of building
materials, plywood (veneer), high-end fittings and cabinets. It finds
applications in various industries including paneling, framing and
flooring. Additionally, the seeds and bark of this species have been
traditionally used in herbal remedies for managing diabetes.
In India, various African nations, as well as Indonesia and Malaysia, Swietenia mahogany is utilized as a medicinal herb [41]. Historically, it has been employed as an antipyretic, bitter tonic and astringent for treating conditions such as malaria, hypertension, diabetes and diarrhea [43,44]. Additionally, it holds significant potential in improving soil quality.
Extraction of Biodiesel
Materials and methods
a. Chemical required: Petroleum ether, ethanol, sodium
hydroxide, potassium hydroxide, phenolphthalein, hydrogen
chloride, n-hexane, sodium thiosulphate, acetic acid, starch
solution, methanol, potassium iodide, chloroform.
b. Apparatus: Burette, beaker, conical flask, petri plates,
burette stand, weighing machine, pipette, spatula, measuring
cylinder, separating funnel, Soxhlet extraction equipment (round
bottom flask, condenser and extractor), magnetic stirrer, fridge,
glass container, thermometer.
c. Sample collection (Swietenia Mahogany seeds): The
seeds of Swietenia Mahogany for this work were collected from a
farmer through the help of Aranya Bhawan, Karnataka state forest
Department. These seeds were collected by the farmer by picking
up from the ground. Later they were separated and dried in sunlight
for peeling process. On the basis of the color and nature, these seeds
were tested for oil content.
d. Seed Preparation: The Swietenia Mahogany seed typically
consists of three parts: the outer shell, inner shell, and the seed
itself. The outer shell acts as a protective covering for the seed,
while the inner shell contains the thin, clustered-shaped seeds. The
seed’s outer shell is divided and the inner shell contains several
seeds. The seeds are brownish-golden in color. They are dried in
sunlight and then peeled to remove the skin. From this brownish
seed, the mahogany seeds are manually extracted. These seeds
are bean-shaped and whitish in color. Figure 3a-3d shows the
mahogany tree leaves and fruits, mahogany seed parts, dried seeds
and mahogany seeds with outer skin peeled.
Figure 3a:Swietenia Mahogany tree leaves and fruits.

Figure 3b:Mahogany seed’s parts.

Figure 3c:Mahogany seeds dried.

Figure 3d:Mahogany seeds outer skin peeled.

e. Oil extraction methods: Specialized techniques such as
Accelerated Solvent Extraction (ASE) and Microwave-Assisted
Extraction (MAE) are employed alongside traditional mechanical,
chemical and enzymatic oil extraction methods to cater to specific
sample types [45].
I. Cold pressed extraction method: This method, known
as expression or cold press method, is one of the oldest ways to
extract oil. It relies on internal friction to generate heat, eliminating
the need for external heat sources. The process involves grinding
raw materials while maintaining minimal temperatures to prevent
damage. The fatty acid portion of the raw materials is punctured and
then mechanically pressed to extract the oil. However, this technique
may result in lower oil quality compared to other methods and not
all raw materials are suitable for cold pressing. Additionally, the oil
yield may not be as high as with other extraction techniques.
II. Accelerated solvent extraction: A novel extraction
technique, referred to as Pressurized Solvent Extraction (PSE), has
emerged for extracting oil from seeds. This method utilizes organic
or aqueous solvents at elevated temperatures and pressures
to facilitate the extraction process. The heightened pressure
prevents the solvent from boiling at temperatures exceeding its
normal boiling point, while elevated temperatures expedite the oil
extraction rate.
III. Microwave-assisted extraction: This method utilizes a
microwave oven to extract plant material at atmospheric pressure,
accelerating the process. The plant material is placed in the reactor
of the microwave oven, where the expansion of plant cells leads
to the release of organic compounds. The internal heating of the
plant material with the solvent occurs within the microwave oven.
Outside the microwave oven, a refrigerant device is positioned to
condense the solvent vapors. Microwaves, which are non-ionizing
radiation, fall within the electromagnetic spectrum between
infrared and radio waves, with frequencies ranging from 300MHz
and wavelengths falling between infrared and radio waves. When
exposed to microwaves, the energy is absorbed, leading to the
migration of ions and dipolar rotation, resulting in molecular
agitation and shocks. These molecular motions and rotations
caused by the electromagnetic energy act as non-ionizing radiation,
but they do not alter the molecular structure.
IV. Ultrasound assisted extraction: Another extraction
method that has gained popularity since 2007 is Ultrasound-
Assisted Extraction (UAE). This technique utilizes ultrasound
waves with frequencies ranging between 20kHz and 100MHz
to enhance mass transfer without significantly altering the
structure and properties of the targeted compounds. Ultrasound
waves induce cavitation, thermal and mechanical effects in the
extraction medium, disrupting cell walls. High-frequency (20kHz)
pulses generate cavitation bubbles, creating local hotspots at
a microscopic scale with high pressure and temperature. Since
UAE can be performed at room temperature, it helps prevent the
decomposition and oxidation of herbal products. UAE has found
widespread application in isolating various natural products.
The three main factors influencing UAE extraction efficiency are
extraction time, solvent composition, and input power. Optimization
of these conditions can be achieved using a response surface model.
Increasing ultrasonic power generally leads to higher seed oil yield,
while increasing temperature tends to decrease it.
f. Operation of soxhlet extraction: In this study, the Soxhlet
method was selected for oil extraction from the seeds due to
its ability to perform multiple extractions. Compared to other
methods, Soxhlet extraction requires less solvent dosage, offers
high efficiency and enables complete extraction. This method has
been widely employed for extracting valuable bioactive compounds
from various natural sources [46]. Its ease of use, continuous
operation and suitability for laboratory settings make it a preferred
choice for oil extraction in this work.
g. Liquid solvent method: This process involves extracting
a solute component from a solid using a liquid solvent. Agitation
of the solvent enhances Eddy diffusion, facilitating the transfer of
substances from the surface to the particles. Soxhlet extractions
were conducted using n-hexane as the solvent due to its higher
oil yield compared to other solvents. In this procedure, 100ml of
solvent was poured into a round bottom flask and 50 grams of
seeds were added. The seeds were finely crushed using a pestle and
mortar (Figure 4a), then wrapped in blotting paper before being
placed in the Soxhlet extractor. The Round Bottom Flask (RB flask)
was initially positioned inside the heating mantle. The extractor was
then connected to the flask, followed by attaching the condenser.
The condenser features one inlet and one outlet hole to allow water
to pass through, aiding in the condensation of vapor, which then
drips onto the sample seeds. Additionally, 150ml of solvent was
added from the upper part of the condenser, which flows into the
extractor itself (Figure 4b).
Figure 4a:Swietenia Mahogany tree leaves and fruits.

Figure 4b:Mahogany seed’s parts.

Once the solvent was boiled and evaporated with the aid of the heating mantle, the vapor condensed and dripped into the extractor, allowing the solvent to return to the bottom flask. This evaporationcondensation process was continued for a minimum of 4 hours. Subsequently, the apparatus was allowed to cool. The resulting cold solvent-seed oil mixture was then transferred into petri plates or a beaker and left overnight to allow for solvent removal. The petri plates or beaker were then weighed both with and without the oil to determine the oil percentage from the seeds.
The percentage of oil obtained was calculated using the formula:
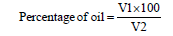
V1- amount of oil gained (after extraction)
V2- amount of seeds used
Characterization of Oil
Acid value and free fatty acid value
To assess the acid value and Free Fatty Acids (FFA) of the oil sample, mixing 25ml of petroleum ether with 25ml of ethanol and adding 4 drops of phenolphthalein indicator is done. Next, combine this solution with 1.03g of the specified oil sample in a conical flask. Titrate the mixture with aqueous 0.1M NaOH until the brownish color vanishes and the solution turns colorless, ensuring thorough shaking throughout the process.

Here M is the base molarity and W is the molecular mass of the base. This procedure should be repeated to obtain more accurate values for the acid value and FFA.
Peroxide value
In a clean and dry boiling tube, 1 gram of oil, along with 1 gram of powdered potassium iodide and 20ml of a solvent mixture comprising 2 parts glacial acetic acid and 1 part chloroform are added. The tube is placed in boiling water to initiate boiling within 30 seconds, allowing it to continue boiling while stirring the contents not to exceed 30 seconds. Afterward, the mixed material is transferred into a flask containing 20ml of potassium iodide solution. The tube is ringed with 25ml of distilled water and titrated with 0.002M sodium thiosulphate solution, using starch as an indicator. A blank titration is performed simultaneously with the sample.

Here V1 = blank titration
V2 =sample titration
W = weight of the sample
N = normality of sodium thiosulphate solute
Iodine Value: A sample weighing between 50 to 100mg of the required oil was placed into a clean and dried iodine flask. Subsequently, a solution consisting of 10ml of 0.1mol/L Cerium Ammonium Nitrate (CAT) in glacial acetic acid was added to the flask and the mixture was left at room temperature for 1 to 2 hours. A separate flask was prepared as a blank by adding an equivalent amount of CAT solution. Following this, 10ml of 10% Potassium Iodide (KI), 50 ml of water and 10ml of 1mol/L Sulfuric Acid (H2SO4) were added to both flasks. The liberated iodine was then titrated against a standard thiosulphate solution, with starch serving as the indicator.

Here, V1 = blank titration
V2 = sample titration
W = weight of sample
M = molarity of thiosulphate
Saponification value
About 0.5-1g of the required oil is weighed into a 100mL round bottom flask or conical flask. Then, 25mL of standard 0.5 N alcoholic KOH solution is added. This solution is refluxed in a steam bath until the solution becomes clear. After cooling, it is diluted to a 250mL standard flask. Next, 25mL of this diluted solution is pipetted out and titrated against 0.5 N HCl, using phenolphthalein as the indicator.
The Saponification value formula:
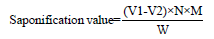
Here, V1 = blank titration
V2 = sample titration
W = weight of sample
M = molecular weight of KOH
Biodiesel
Conversion of oil into biodiesel
The transesterification process is recognized for its eco-friendly nature and its feasibility under mild operating conditions. It serves as a method to produce biodiesel from various feedstocks, valued for its capability to generate high-quality fuel. In transesterification, a triglyceride (fat/oil) undergoes a reaction with alcohol, resulting in the formation of esters and glycerol. A triglyceride comprises a glycerin molecule with three long-chain fatty acids attached. The characteristics of these facts are dictated by the nature of the attached fatty acids, consequently influencing the properties of the resulting biodiesel. Transesterification is a reversible process that typically transpires through the mingling of reactants. Nonetheless, the conversion rate can be hastened with the aid of a catalyst, which could be a potent acid or base. Furthermore, the introduction of a small quantity of alcohol aids in tilting the equilibrium towards the generation of fatty acid alkyl esters and glycerol.
Transesterification is the process of converting acylglycerols (glycerides), such as Monoacylglycerols (MAG), Diacylglycerols (DAG) and Triacylglycerols (TAG), along with Free Fatty Acids (FFA), into biodiesel or Fatty Acid Methyl Ester (FAME). Triglycerides are especially preferred for biodiesel production due to their high yield. Among the various techniques, homogeneous alkaline transesterification is notable for its simplicity and superior catalytic activity compared to alternative methods.
In a 150-milliliter flask, 14 milliliters of methanol were precisely measured and added. Sodium hydroxide pellets (0.50 grams), previously crushed using a dry mortar and pestle, were transferred into the flask containing the methanol. A stir bar was then placed inside the flask, and the flask was positioned on a magnetic stirrer. Stirring was maintained for 5-10 minutes until complete dissolution of the NaOH was achieved. Subsequently, the mass of the oil was determined at 50 °C and fifty milliliters of seed oil were measured and carefully added to the flask. The flask was then heated to 50 °C for 30-45 minutes while ensuring continuous stirring to prevent the mixture from stratifying into two layers. Once the mixture achieved homogeneity, it was carefully transferred into a separatory flask while still warm. The separatory flask was then allowed to cool until distinct layers formed.
In the separatory flask, the biodiesel or oil methyl ester forms the upper layer, while glycerol typically forms the lower layer (Figure 5a & 5b). Due to the low solubility of glycerol esters, they can be easily and quickly separated from the separatory flask by drainage (Figure 6). Following the addition of 10 milliliters of water to the flask, the biodiesel underwent a washing process. The mixture was gently swirled for approximately 1 minute to dissolve any residual methanol, glycerin, sodium hydroxide and soap. Subsequently, the mixture was allowed to settle and the lower layer containing impurities was carefully discarded. The biodiesel layer was then collected in a dry beaker.
Figure 5:a. Oil solution heating on the water bath. b. The upper layer is biodiesel and lower layer is glycerin in separating flask.
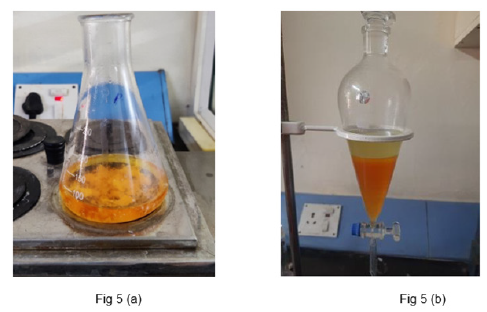
Figure 6:Biodiesel obtained.

To eliminate any remaining traces of water from the biodiesel, 0.5 grams of anhydrous sodium sulfate were added to the biodiesel and gently swirled for a period of time. The dried biodiesel was subsequently transferred into a clean, dry, pre-weighed measuring cylinder. The mass and volume of the biodiesel were accurately measured and the percentage yield was determined using the appropriate equation.
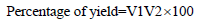
In which, V1 = after conversion quantity of biodiesel received
and
V2 = for conversion the extent of oil used
Results & Discussion
Percentage of oil obtained from Soxhlet extraction method
Based on studies conducted on Swietenia Mahogany seeds, it has been determined that the seeds yield approximately 38-42% oil. This oil yield surpasses that of other sources like soybean and rapeseed, rendering it a cost-effective option for oil recovery. The enhancement in oil extraction from mahogany seeds can be ascribed to modifications in the extraction method. Initially, methanol was employed as a solvent in the Soxhlet extraction method, but it yielded unsatisfactory results. The oil obtained exhibited a wax-like consistency, likely due to polarity differences.
Subsequently, n-hexane was utilized as a solvent, resulting in a successful extraction without the wax-like consistency. The oil obtained possessed a whitish color akin to coconut oil, along with a bitter taste and a plant-like odor at room temperature. This method yielded approximately 40% of oil, a notable value compared to other non-conventional seed oils. Research indicates that crops with high oil content, such as Swietenia Mahogany, hold promise for biodiesel production, providing a potential alternative to traditional sources.
Characterization of oil
Acid value and free fatty acid value: Examining the acid value of the oil offers valuable insights into potential spoilage, hyper-enzymatic activity and the free fatty acid content of the material. Prior studies have suggested that oils with elevated acid values might deactivate the catalyst utilized in transesterification processes. In the case of Swietenia Mahogany seed oil, the process yielded an acid value of 6.6 and a free fatty acid value of 3.3, indicating a high amount of acid present in the oil. The age of the oil can be determined by observing the increase in acid number over time, attributed to hydrolysis with moisture.
Peroxide value: The peroxide value of oil indicates the extent of oxidative rancidity and deterioration. It reflects the susceptibility of the oil to oxidation, based on the range of double bonds present, which correlates with its unsaturation level and places it in the nondrying group. Different pre-treatments significantly affect the yield and peroxide value of the extracted oils. For Swietenia Mahogany seed oil, a peroxide value of 2.5 was recorded, indicating a highquality oil with good preservation status. The peroxide value, which reflects the concentration of peroxide in oil or fats, serves as a valuable metric for evaluating the extent of spoilage. and oxidation in diesel engines, as well as determining the drying property of the oil.
Iodine value: The iodine value of oil indicates the extent of unsaturation and affects the deposition and oxidation of the diesel engine. For Swietenia Mahogany seed oil, an iodine value of 120 was obtained, indicating a higher unsaturation level. A higher iodine value suggests greater unsaturation in the oil, making it suitable for soap making. Additionally, this oil is of the non-drying type, meaning it does not undergo significant drying or hardening upon exposure to air.
Saponification value: The Saponification value of an oil indicates its propensity to form soap during the transesterification process, indicating the presence of higher fatty acids in higher proportions.
For Swietenia Mahogany seed oil, a determined Saponification value of 90.5 was recorded. This value falls within an average range, neither too low nor too high compared to other non-edible oils suitable for biodiesel production. A lower Saponification value suggests a higher proportion of higher fatty acids in the oil, making it suitable for soap preparation. Additionally, the oil exhibits potential for biodiesel production due to its moderate Saponification value. Table 1 presents the characteristic values of different analytical parameters investigated for the oil and Table 2 illustrates the characteristics of the biodiesel prepared in this study.
Table 1:Analytical data obtained for various components.
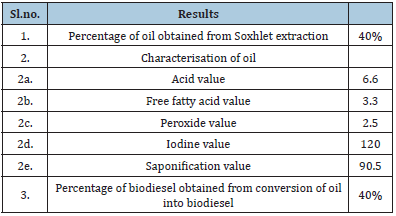
Table 2:Characterization of biodiesel.

Conversion of oil into biodiesel
Biodiesel, an alternative fuel derived from plant oils, undergoes a chemical process called transesterification to convert the oil into its corresponding fatty ester in the presence of a catalyst. This method transforms esters from long-chain fatty acids into mono alkyl esters, commonly referred to as Fatty Acid Methyl Esters (FAME). This process effectively decreases the oil’s viscosity, rendering it suitable for use as a fuel.
During the transesterification process, typically 15% methanol and 5% sodium hydroxide are added to the required oil on a mass basis to facilitate the formation of fatty acid methyl esters from seed oils. This is an equilibrium reaction that requires additional alcohol to drive the process to completion. Once the conversion is complete, the solution is separated into two layers in a separatory flask, with glycerin settling in the lower layer and biodiesel in the upper layer. The water content in the upper layer is further reduced by adding anhydrous sodium sulphate.
The resulting biodiesel from Swietenia Mahogany seed oil is pale yellow in color, with a bitter taste and a smell reminiscent of plant material. The yield obtained from this process is 40%, which is a notably good value. The glycerin obtained as a by-product can be utilized in soap industries, providing additional economic opportunities. Due to limitations caused by the pandemic and individual financial constraints, the biodiesel production was limited to a small scale in the laboratory. However, with greater investment and support from sponsors or investors, larger-scale production could be undertaken, allowing for further testing and characterization of the biodiesel obtained.
LC-MS Analysis
LC-MS is a sophisticated analytical method that integrates Liquid Chromatography (LC) for separation and Mass Spectrometry (MS) for detection. This technique enables accurate analysis of target compounds by segregating them according to their physical properties and subsequently identifying them based on their mass. In this research, LC-MS analysis was conducted using a XEVO-G2XSQTOF#YFA1548 MassLynx 4.1 SCN949 instrument at Vigyan Bhawan, University of Mysore. This advanced technique facilitated the determination of the molecular weight and fatty acid composition of Mahogany Oil, along with the composition of the biodiesel produced from it.
LC-MS is highly prized for its sensitivity, selectivity and accuracy, rendering it well-suited for detecting even trace amounts of diverse analytes like drug metabolites, pesticides, food adulterants and natural product extracts. Through the application of LC-MS, we gained comprehensive insights into the chemical composition of Mahogany Oil and its biodiesel. The results are illustrated in Figure 7a-7d, where peaks were identified using retention times, fragmentation patterns and comparison with established libraries (Table 3). Furthermore, authentication was conducted via molecular weight estimates and supplementary information from the Phenol-Explorer database. Four major peaks were recognized, each representing distinct classes of compounds (Table 3), including phenolic acid, flavonoid, ester, alkaloid and fatty acid categories (Table 4). Structural characterization of phenolic acid mainly depended on their MS spectra, offering valuable insights into the composition and properties of the analyzed substances.
Figure 7a:LC data of MSO.

Figure 7b:LC data of MSO.
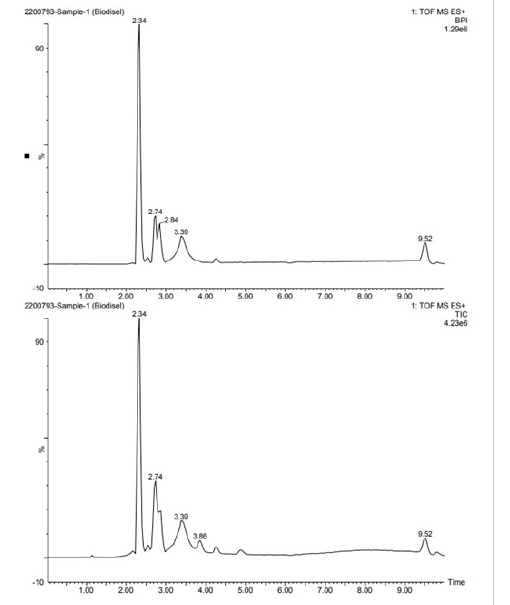
Figure 7c:LC data of MSO.
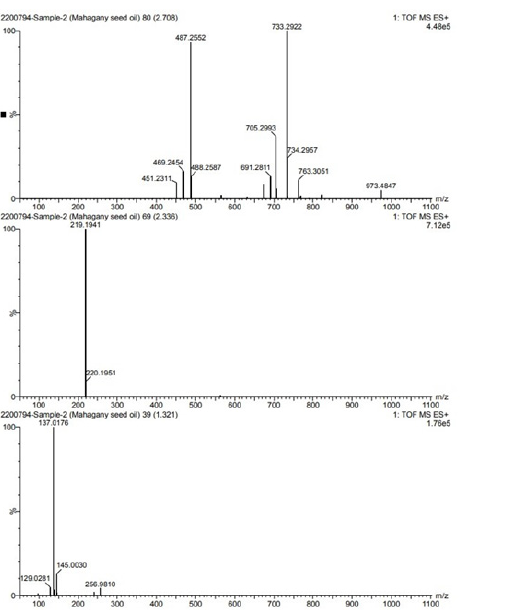
Figure 7d:LC data of MSO.

Table 3:List of detected compounds from mahogany seed oil observed from LC-MS with their retention time, mass spectral data.

Table 4:List of detected compounds from Biodiesel observed from LC-MS with their retention time, mass spectral data.

Conclusion and Future Prospect
The study highlights the potential of Swietenia mahogany seeds as a non-edible source for biodiesel production. It emphasizes the need to shift towards second-generation biofuels to mitigate the food vs. energy dilemma. While the physiochemical qualities of Swietenia mahogany seed oil make it promising for biodiesel production, further research is required to optimize extraction processes and reduce manufacturing costs. Additionally, advancements in engine systems are crucial for seamless integration of biofuels into the transportation sector [47]. Addressing logistical challenges, such as farmer-biodiesel facility connections and marketing gaps, is essential. Moreover, government support and policy incentives can bolster biofuel production. Biomass, particularly through pyrolysis, presents a viable avenue for liquid fuel production, offering benefits in storage, transportation and application versatility. Future efforts should focus on technological advancements in pyrolysis to maximize biofuel output and competitiveness with fossil fuels in various applications [48-53].
Acknowledgement
The authors are thankful to all those who helped in the collection and experimental studies of MSO and biodiesel investigation.
References
- Demirbas A, Bafail A, Ahmad W, Sheikh M (2016) Biodiesel production from non-edible plant oils. Energy Exploration & Exploitation 34(2): 290-318.
- Donate PM (2014) Green synthesis from biomass. Chemical and Biological Technologies in Agriculture 1(1): 1-8.
- Dolan RH, Wallington TJ, Anderson JE (2024) Large decreases in tailpipe criteria pollutant emissions from the US light-duty vehicle fleet expected in 2020-2040. Environmental Science & Technology.
- Turoń K (2020) Hydrogen-powered vehicles in urban transport systems-current state and development. Transportation Research Procedia 45: 835-841.
- Rana SM, Haque MA, Poddar S, Sujan SM, Hossain M, et al. (2015) Biodiesel production from non-edible mahogany seed oil by dual step process and study of its oxidation stability. Bangladesh J Sci Ind Res 50(2): 77-86.
- Altın R, Cetinkaya S, Yücesu HS (2001) The potential of using vegetable oil fuels as fuel for diesel engines. Energy Conversion and Management 42(5): 529-538.
- Kuppili SK (2016) Biodiesel properties and characterization of particulate matter emissions from TARTA buses fueled by B20 biodiesel. Doctoral Dissertation, University of Toledo, Ohio, USA.
- Al-Dhubabian AA (2005) Production of biodiesel from soybean oil in a micro scale reactor.
- Hassan MH, Kalam MA (2013) An overview of biofuel as a renewable energy source: Development and challenges. Procedia Engineering 56: 39-53.
- Huang D, Zhou H, Lin L (2012) Biodiesel: An alternative to conventional fuel. Energy Procedia 16: 1874-1885.
- Fakhfakh J, Ben-Youssef S, Naushad M, Allouche N (2019) Different extraction methods, physical properties and chemical composition of date seed oil. Sustainable Agriculture Reviews 34: Date Palm for Food, Medicine and the Environment 125-153.
- Zulqarnain M, Yusoff MH, Nazir MH, Zahid I, Ameen M, et al. (2021) A comprehensive review on oil extraction and biodiesel production technologies. Sustainability 13(2): 788.
- Gunawan TA, Williamson I, Raine D, Monaghan RF (2021) Decarbonising city bus networks in Ireland with renewable hydrogen. International Journal of Hydrogen Energy 46(57): 28870-28886.
- Shekhawat D, Spivey JJ, Berry DA (2011) Fuel cells: Technologies for fuel processing.
- Khan TY (2020) A review of performance-enhancing innovative modifications in biodiesel engines. Energies 13(17): 4395.
- Mohanty P, Pant KK, Naik SN, Das LM, Vasudevan P (2011) Fuel production from biomass: Indian perspective for pyrolysis oil.
- Bethoux O (2020) Hydrogen fuel cell road vehicles and their infrastructure: An option towards an environmentally friendly energy transition. Energies 13(22): 6132.
- Guo M, Song W, Buhain J (2015) Bioenergy and biofuels: History, status and perspective. Renewable and Sustainable Energy Reviews 42: 712-725.
- Yusup S, Chuah LF, Bokhari A (2016) Physio-chemical studies of locally sourced non-edible oil: Prospective feedstock for renewable diesel production in Malaysia. Procedia Engineering 148: 451-458.
- Dahman Y, Dignan C, Fiayaz A, Chaudhry A (2019) An introduction to biofuels, foods, livestock and the environment. Biomass, Biopolymer-Based Materials and Bioenergy 241-276.
- Amiri H (2020) Recent innovations for reviving the ABE fermentation for production of butanol as a drop-in liquid biofuel. Biofuel Research Journal 7(4): 1256-1266.
- Sayyed SR, Gitte BM, Joshi SD, Dharmadhikari HM (2013) Characterization of biodiesel: A review. Int J Eng Res Technol 2(10).
- Carriquiry MA, Du X, Timilsina GR (2010) Second-generation biofuels: Economics and policies. Energy Policy 39(7): 4222-4234.
- Baskar G, Kalavathy G, Aiswarya R, Selvakumari IA (2019) Advances in bio-oil extraction from nonedible oil seeds and algal biomass. Advances in Eco-Fuels for a Sustainable Environment 187-210.
- Fukushima K, Masuda M, Tani Y, Shiga K (2011) The socio-economic role of tree-borne oilseeds in rural livelihood: A case study in Karnataka State, India. Tropics 20(3): 87-95.
- Gashaw A, Lakachew A (2014) Production of biodiesel from non-edible oil and its properties. International Journal of Science, Environment and Technology 3(4): 1544-1562.
- Kumar N, Sonthalia A, Pali HS, Sidharth (2019) Next-generation biofuels-opportunities and challenges. Innovations in Sustainable Energy and Cleaner Environment 171-191.
- Perona JJ (2017) Biodiesel for the 21st century renewable energy economy. Energy LJ 38: 165.
- Abnisa F, Daud WW, Husin WN, Sahu JN (2011) Utilization possibilities of palm shell as a source of biomass energy in Malaysia by producing bio-oil in pyrolysis process. Biomass and Bioenergy 35(5): 1863-1872.
- Janda K, Kristoufek L, Zilberman D (2012) Biofuels: Policies and impacts. Agricultural Economics 58(8): 372-386.
- Can S, Letschert VE, Mcneil MA, Zhou N, Sathaye J, et al. (2018) Residential and transport energy use in India: Past trend and future outlook. Building and Environment 142: 66.
- Kumar A, Sharma S (2011) Potential non-edible oil resources as biodiesel feedstock: An Indian perspective. Renewable and Sustainable Energy Reviews 15(4): 1791-1800.
- Shastri CM, Bhat DM, Nagaraja BC, Murali KS, Ravindranath NH (2002) Tree species diversity in a village ecosystem in Uttara Kannada district in Western Ghats, Karnataka. Current Science 82(9): 1080-1084.
- Daniel JN, Hegde NG (2007) Tree-borne oilseeds in agroforestry. In Proceedings of the National Seminar on Changing Global Vegetable Oils Scenario: Issues and Challenges before India. Indian Society of Oilseeds Research, Hyderabad, India, pp. 263-276.
- Dhyani SK, Devi SV, Handa AK (2015) Tree borne oilseeds for oil and biofuel. Technical Bulletin 50.
- Arora G, Kumar D (2015) Tree-Borne Oilseed (TBOs): Competitive source for biodiesel in India. Journal of Biofuels 6(1): 15.
- Ramachandra TV, Chandran MD, Bhat SP, Aithal BH, Rao GR, et al. (2013) Status of forest in Shimoga district, Karnataka, Sahyadri conservation series 23. ENVIS Technical Report: 53. ENVIS-Environmental Information System, Centre for Ecological Sciences, Indian Institute of Science, Bangalore, India.
- Chukwujike JL, Ewulonu MC, Chukwujike IC, Okolie PC (2019) Physico-chemical analysis of pyrolyzed bio-oil from swietenia macrophylla (mahogany) wood. Heliyon 5(6): e01790.
- Chukwuneke JL, Sinebe JE, Ugwuegbu DC, Agulonu CC (2016) Production by pyrolysis and analysis of bio-oil from mahogany wood (swietenia macrophylla). British Journal of Applied Science & Technology 17(4): 1-9.
- Kadarwati S, Qurrochman T, Kurniawan C, Jumaeri, Kasmui (2020) Feasibility study on the utilization of mahogany (swietenia macrophylla king) wood as a raw material in the bio-oil production. In Journal of Physics: Conference Series 1567(2): 022029.
- Krisnawati H, Kallio MH, Kanninen M (2011) Swietenia macrophylla king: Ecology, silviculture and productivity. CIFOR, Bogor, Indonesia.
- Barros P, Carvalho JO, Silva TP, Oliveira LR, Costa JP (2013) Ecology and silviculture of mahogany (swietenia macrophylla king) in the Western Brazilian amazon.
- Majid MA, Rahman IM, Shipar MA, Uddin MH, Chowdhury R (2004) Physico-chemical characterization, antimicrobial activity and toxicity analysis of swietenia mahagoni seed oil. International Journal of Agriculture and Biology 6(2): 350-354.
- Yadav S (2018) Antibacterial activity of mahogany (swietenia mahogany (L.) Jacq.) seeds oil and analysis of their fatty acids. 6(1): 570-582.
- Ramos JH, Hernandez A, Cuevas XG, Ángel LM, Urías JC, et al. (2018) Compatible commercial taper-volume system for swietenia macrophylla king (mahogany) in quintana roo, Mexico. Wood and Forests 24(3): 1-11.
- Graham PB (2018) The study of biodiesel production using different soxhlet extraction method. International Journal on Recent Technologies in Mechanical and Electrical Engineering 5(4): 12-15.
- Montaña JR, Diez AJ, Varga ME, Téllez SA (2016) Transportation sector energy consumption. International Sheep Farming. Mexican Association of Sheep Farming Specialists, pp. 127-137.
- Abdullah GZ, Abdulkarim MF, Salman IM, Ameer OZ, Chtneni M, et al. (2010) HPLC method modification and validation for quantification of ibuprofen. International Journal of Advances in Pharmaceutical Sciences 1(4).
- Jitan SA, Alkhoori SA, Yousef LF (2018) Phenolic acids from plants: Extraction and application to human health. Studies in Natural Products Chemistry 58: 389-417.
- Danbature WL, Aliyu J, Adamu HM (2015) Production of methyl ester from mahogany (khaya senegalensis) seed oil. Journal of Chemistry and Chemical Sciences 5(9): 489-499.
- Datta A, Hossain A, Roy S (2019) An overview on biofuels and their advantages and disadvantages. Asian Journal of Chemistry 31(8).
- Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S (2009) World agroforestry center: Swietenia mahagoni. Agroforestry Database, pp. 1-5.
- Rai V, Patel SK, Muthuraj M, Gandhi MN, Das D, et al. (2021) Systematic metabolome profiling and multi-omics analysis of the nitrogen-limited non-model oleaginous algae for biorefining. Biofuel Research Journal 8(1): 1330-1341.
© 2024 Manager Rajdeo Singh, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






