- Submissions

Full Text
Significances of Bioengineering & Biosciences
Synthesis and Characterization of the New Fac-and Mer-[M (Caf)3(SCN)3],OH2
El Amane M*, El Hamdani M, Bouhdada M and Kennouche Y
Université Moulay Ismail, Morocco
*Corresponding author: Amane MEL, Université Moulay Ismail, Faculté des sciences, BP11201 Zitoune, Meknes Morocco
Submission: September 15, 2017; Published: December 05, 2017

ISSN 2637-8078Volume1 Issue1
Abstract
New fac-and mer-[M(caf)3(SCN)3]OH2 caffeine complexes where caf = caffeine, SCN= thiocyanato and M= Cr(III), Fe(III) and Ru(III) were synthesized in simple reactions of chloride MCl3, H2O ; M= Cr(III), Fe(III) and Ru(III) with potassium thiocyanate in ethanol solution. The infrared and electronic spectral data of the complexes [M(caf)3(SCN)3]OH2 suggest that the caffeine is coordinate through the nitrogen N9 and the thiocyanato behave a monodentate legend with sulphur atom donor towards metal ions. On the basis of the spectral data, the FT-IR, UV-Visible and EPR suggested the mer- and fac-octahedral complexes.
Keywords: Caffeine; Mer; Fac; Complexes; Thiocyanato; Infrared; UV-Visible; EPR; Molar conductance
Introduction
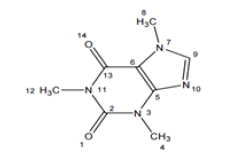
Transition metal Complexes have received great attention for many years, because of their catalytic, biological activity, essentially due to interaction with their heavy metal ions, bonding through nitrogen and oxygen. The interest in metal complexes of Purina and development of the field of bioinorganic chemistry have increased substantially, since it has been reorganized that many of the complexes may serve as models for biologically important species, i.e. the caffeine is a strong antioxidant that prevent DNA damage. The interaction between nucleic acid and metal ions constitutes a field with multidisciplinary characters such as the bioinorganic chemistry or inorganic biochemistry. N-heterocyclic carbines (NHCs) have been isolated in 1991 [1], and have become ubiquitous in coordination chemistry particularly in the field of catalysis [2-7]. In more recent years, metal NHCs, have shown promise as antimicrobial (AgI-NHCs) and as antitumor (Pd (II), Cu (II), Au (I) and Rh (II) NHCs) agents [8-12]. Caffeine (Figure 1) is the more thoroughly studied methylxanthines to date. Caffeine was first isolated from sea and coffee in the early 1820 [13-15]. Caffeine is widely perceived as a central nervous system (CNS) stimulant. It acts like a brain cortex stimulant and is usually sought for by those looking for a general sense of mental energy with increased awareness and wakefulness improved clear thinking and attenuated fatigue. It was reported as enhancing a wide range of exercise activities from those relying on explosive strength to short term [16]. Caffeine has a centrosymmetric Cs point group. Its number of normal modes of vibration can be distributed as r , = vib27 A' + 12 A''. It's known from the literature that the coordination of the caffeine with nitrogen and oxygen atoms which is accompanied by elimination of the mirror plane ah and by a whole series of changes in the infrared spectrum [17]. We report here the synthesis and characterization of some new fac- and mer- [M(caf)3X3]; M= Cr(III), Fe(III) and Ru(III) containing caffeine and thiocyanato as legends. The synthesized complexes are characterized by molar conductance, infrared, EPR and electronic spectroscopic analysis.
Figure 1: Structure of caffeine (1,3,7-trimethyl-3,7-dihydro-purine-2,6-dione).
Materials and Methods
All chemicals were obtained from commercial sources and were used without purifications: (FeCl3, 6H2O BDH; CrCl3^ 6H2O BDH; RuCl3(H2O)3 Sigma Aldrich), caffeine Sigma Aldrich, potassium thiocyanate Sigma Aldrich, Ethanol and DMSO Sigma Aldrich. Infrared spectra were recorded as KBr pellets on a JASCO FT-IR 660 plus spectrophotometer in the range of 4000-400cm-1 at 298K while the UV-Visible spectra were obtained on a Shimadzu UV-1800 Spectrophotometer. The EPR spectrum was recorded on a conventional X band Burke ER 200D spectrometer operating at 9.5GHz. Conductivity measurements were performed at 25 °C in DMSO using Hatch HQ430d flexi.
Synthesis of the fac- and mer- caffeine complexes [M(caf)3(SCN)3OH2, where caf = caffeine and M= Cr(III), Fe(III) and Ru(III)
To a solution of caffeine (3mmol) and potassium thiocyanate KSCN (3mmol) in ethanol, was added a solution of metal salts (Fe(III) and Cr(III)) (lmmol) in ethanol. The obtained solution was refluxed for 4h, after which the solution is concentrated, filtered and dried.
Results and Discussion
Characterization of fac- and mer- caffeine complexes [M(caf)3(SCN)3]OH<2 Where caf = caffeine and M= Cr(III), Fe(III) and Ru(HI)
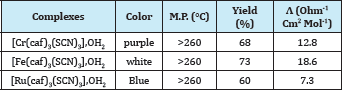
The analytical data and some physical properties of the fac- and mer- caffeine complexes [M(caf)3(SCN)3] OH2; M= Cr3+, Fe3+ and Ru3+ are collected in Table 1. The complexes are stable in air, soluble in DMSO and DMF. Molar conductance values of the fac- and mer- caffeine complexes in DMSO (10-3M solution at 25 °C) were (7.3-18.6)Ω-1cm2 mol-1 indicating, their non-electrolytic nature [18] (Table 1).
Table 1: Physico-chemical data of the fac-and mer-caffeine complexes.
Infrared spectra
In order to study binding mode of the caffeine and thiocyanato ion to Chromium(III), Ruthenium(III) and Iron (III) in the new complexes Figure 2 &Table 2
Figure 2: Infrared spectrum of [Fe(caf)3(SCN)3],OH2 in KBr.
Table 2 Spectral data of the fac and mer-caffeine complexes [M(caf)3(SCN)3],OH2; M=Cr3+, Fe3+ and Ru3+
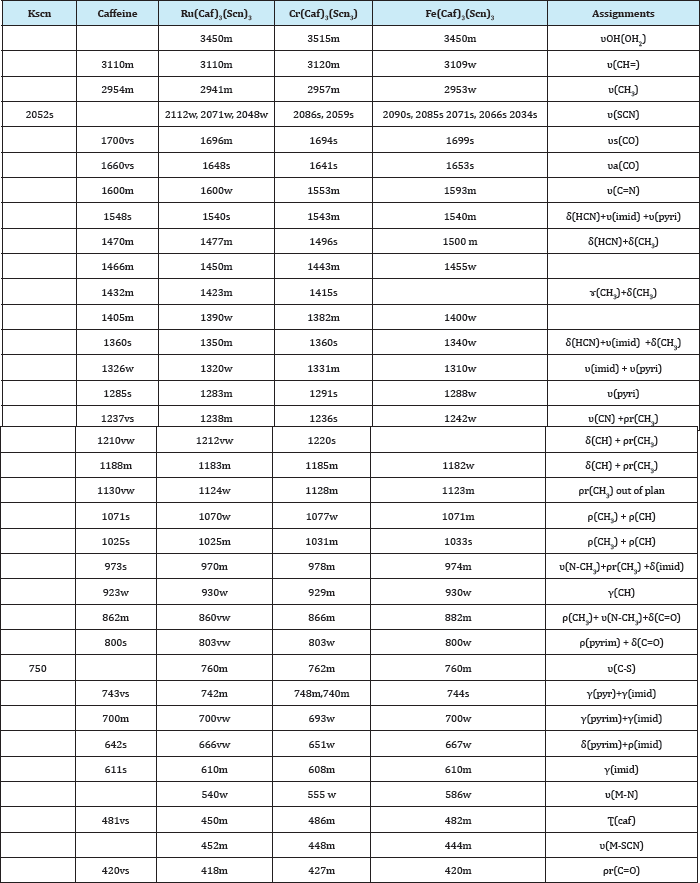
The infrared spectrum of the free caffeine [19] (Table 2) is characterized by absorption peaks at 3110cm'1 and 2954cm'1 assigned to the v(C-H) stretching vibrations in imidazole ring and v(CH3) stretching vibration in imidazole and pyrimidine rings respectively. A strong bands are observed at 1700cm-1 and 1660cm- 1 which are characteristic of the asymmetric and symmetric v(C=O) stretching vibration in pyrimidine ring respectively. The v(C=C) and v(C=N) vibrations of the free caffeine that occur at 1600cm" 1 and 1548cm’1 for imidazole ring. The different modes of the methyl bending and rocking are observed in (1470-1025)cm-1 range. In the infrared spectra of all fac-and mer-caffeine complexes, the characteristic bands of the free caffeine are shifted to lower frequencies. The v(C-H) stretching vibrations in imidazole ring and methyl groups in pyrimidine ring are shifted to higher frequencies with the higher intensity in the fac-and mer- caffeine complexes. The stretching frequencies of the symmetric and asymmetric vs(C=O), vas(C=O), v(C=C), v(C=N), 5HCN which appeared in (1694- 1699)cm-1, (1641-1655)cm-1, (1553-1598)cm-1 and (1540-1550) cm-1 ranges respectively [22,23] are shifted to lower frequencies in the fac-and mer-caffeine complexes. The methyl vibrations attached with nitrogen bending and rocking atom in the side chain N-CH3 vibration which are found to be observed around (1340-1496) cm-1 and (978-1077)cm-1 respectively are shifted in the fac-and mer-caffeine complexes. Bands of variable intensity are observed in the region (1100-1000)cm-1 due to in plane deformation vibration in the imidazole and pyrimidine rings. The medium and strong vibrational bands in the regions (974-978)cm-1 and (800- 803)cm-1 are assigned to the 5 (imid) +v (N-C) and p (pyrimid) +5 (C=O) respectively [24,25]. However, during the complexes formation of the caffeine, the imidazole ring gets more frequency changes with higher intensity (Table 2), suggesting that the caffeine coordinate through the imidazole vibration N9 atom, and acting as monodentate ligand [26]. The thiocyanato ligand in the fac- and mer- caffeine complexes absorbed at five frequencies [27]. Strong vibrations in the (2034-2122)cm-1 range for [Fe(caf)3(SCN)3] and [Ru(caf)3(SCN)3] are due to v(SCN) stretching vibration and may attributed to the presence of the thiocyanato in the coordination sphere of these complexes. The multiplicity of the band indicated two isomers which are distinguishable by the infrared spectroscopy [27]. The five absorptions are characteristic for mer and fac isomers in the new complexes. The thiocyanato ligand can coordinate the metal through either the nitrogen or the sulphur. The free thiocyanate ion absorbs at about 2052cm-1 and 750cm-1 which are due to v (SCN) and v (C-S) stretching vibrations [28].These peaks are shifted to higher frequencies in the complexes suggesting the coordination through the sulphur atom (M-SCN). Therefore, the spectra show five bands in (2034-2090) cm-1 range attributed to two isomers mer [M(caf)3(SCN)3] (2A1+B1) with three actives bands in IR (2A1+B1) and the fac isomer has two actives bands in IR (A1+E) [27]. New bands in (555-586)cm-1 range are assigned to u (M-N) vibration [22].
Electronic spectraTable 3: UV-Visible data of the fac-and mer-caffeine complexes [M(caf)3(SGN)3]GH2; M= Gr3+;, Fe3+; and Ru3+.
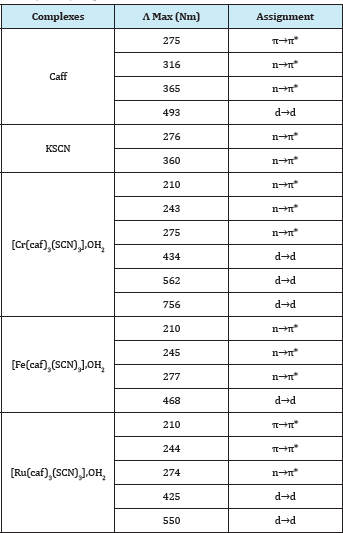
Figure 3: Electronic spectrum of [Cr(caf)3(SCN)3],OH2, in DMSO.
All absorptions were fully assigned in Table 3 and the electronic spectrum of [Cr(caf)3(SCN)3] is shown in Figure 3. The UV-Visible data ofthe thiocyanato and caffeine ligands [29] were compared with those of the fac- and mer-caffeine complexes [M(caf)3(SCN)3],OH2 ; M= Cr3+, Fe3+ and Ru3+. The electronic spectra of the thiocyanato and caffeine ligands [29] show the absorption bands in the UV region can be attributed to and n^n* transitions which were shifted to higher wavelength upon coordination. Therefore, new bands at longer wavelength observed in (366-756)nm range may be assigned to d-d transitions.
EPR spectra
Figure 4: EPR spectrum of [Fe(caf)3(SCN)3]OH2 at room temperature on X band in solid state
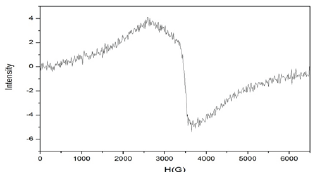
The X-band EPR spectrum of the complex [Fe(caf)3(SCN)3] OH2, in the solid state recorded at room temperature are shown in Figure 4. ESR spectrum of the complex shows only an intense and broad signal without hyperfine splitting (giso=2.151). The shape of the spectrum is consistent with octahedral environment around Fe (II) ion and the higher g value for the investigated complex, when compared to that of free electron (g = 2.0023) revealing an appreciable covalence of metal-ligands (M-N9 and M-SCN) bonding characteristic of octahedral stereochemistry [30-32] (Table 3).
Conclusion
New fac- and mer- caffeine complexes [M(caf)3(SCN)3],OH2; where caf=caffeine, M= Cr3+, Fe3+, Ru3+ were synthesized and characterized on the basis of molar conductance, infrared, UV- Visible EPR. The spectroscopic data suggest that the M (III) caption lies on a fac-and mer-arranged octahedral coordination geometry provided by sulfur atoms of three thiocyanato ligands and nitrogen atoms of three coordinated caffeine molecules and hydration water molecule.
References
- Arduengo AJ, Harlow RL, Kline M (1991) A stable crystalline carbine. J Am Chem Soc 113(1): 361-363.
- Schwab P, France MB, Ziller JW, Grubbs RH (1995) Angew Chem Int Ed 34: 2039.
- Trnka TM, Grubbs RH (2001) The development of L2X2Ru=CHR olefin metathesis catalysts: an organometallic success story. Acc Chem Res 34(1): 18-29.
- Herrmann WA (2002) N-heterocyclic carbenes: a new concept in organometallic catalysis. Angew Chem Int Ed Enql 41(8): 1290-1309.
- Crudden CM, Allen DP (2004) Coord Chem Rev 248: 2247.
- Snead DR, Seo H, Hong S (2008) Curr Org Chem 12: 1370.
- Fremont P, MarionN, NolanSP (2009) Carbenes: Synthesis, properties, and organometallic chemistry. Coord Chem Rev 253: 862-892.
- Anna VR, allepogu RP, Zhou ZY, Kollipara MR (2012) Inorg Chim Acta 387: 37-44.
- Lin IJB, Vasam CS (2007) Coord Chem Rev 251(5-6): 642-670.
- Coleman KS, Chamberlayne HT, Turberville S, Gren MLH, Cowley AR, et al. (2003) Dalton Trans 14: 2917-2922.
- Wang HMJ, Lin IJB (1998) Facile Synthesis of Silver(I)-Carbene Complexes. Useful Carbene Transfer Agents. Organometal 17(5): 972975.
- Wang JW, Song HB, Li QS, Xu FB, Zhang ZZ, et al. (2005) Inorg Chim Acta 358(13): 3653-3658.
- Daly JW (2007) Caffeine analogs: biomedical impact. Cell Mol Life Sci 64(16): 2153-2169.
- Kato M, Mizuno K, Crozier A, Fujimura T, Ashihara H (2000) Caffeine synthase gene from tea leaves. Nature 406: 956-957.
- Kato M, Mizuno K, Fujimura T, Iwama M, Irie M, et al. (1999) Plant Physiol 120: 586-597.
- Chmidt B, Roberts RS, Davis P (2006) Caffeine therapy for apnea of prematurity. N Engl J Med 354(20): 2112-2121.
- Martinez CE , Lozada MC, Gnecco D, Enriquez RG, Reynolds W, et al. (2012) J Chem Crys 42(8): 797-802.
- BashSF, Padusha MSA (2015) Global J Res Analysis 2277(4): 1-3.
- Jenniefer SJ, Muthiah PT (2013) Supramolecular architectures and structural diversity in a series of lead (II) Chelates involving 5-Chloro/ Bromo thiophene-2-carboxylate and N, N'-donor ligands. Chem Central J 7(1): 139.
- Carter TJ, Wilson RE (2015) Coordination Chemistry of Homoleptic Actinide(IV)-Thiocyanate Complexes. Chem Eur J 21(44): 15575-15582.
- Schutte CJH (1963) A J Phy Sci pp. 525-530.
- Ucun F, Saglam A, Guclu V (2007) J spectrochim ACTA PARTA 67: 342349.
- Edwards HGM, Faewell DW, De Oliveira LFC, Alia JM, le Hyaric M, et al. (2005) Anal Chimi Acta 532: 177.
- David L, Cozar O, Forizs E, Craciun C, Ristoiu D, et al. (1999) Spectrochim Acta Part A 55: 2559-2564.
- Hashimi SMA, Jaboori MEA, Azawi SASA (2006) Um-Salama Sci J 3(3): 517-527.
- Amane MHL, Kennouche Y (2014) Int J Chem Tech Res 6(1): 495-508.
- Ghaleb A, Amane MHL, Kennouche Y, Hamdani HEL, Bouhdada M, et al. (2016) J Appl Chem 5(3): 670-677.
- Nakamoto K (1986) Infrared and Raman spectra of inorganic and coordination compounds, Fourth Edition. J W Son Inc.
- Amane MHL, Kennouche Y, Bouhdada M, Ahmami M, Hadad M (2016) J Mar Chim Heterocyclic 15(1): 41-49.
- Rajasekar K, Ramachandramoorthy T, Paulraj A (2012) Res J Pharma Sci 1(4): 22-27.
- Greenberg J (1963) J Chem Phys 39(11): 3158-3159.
- Dolaz M, McKee V, Gölcü AE, Tümer M (2009) Spectrochim Acta Part A 71: 1648-1654.
© 2017 El Amane M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






