- Submissions

Full Text
Research in Pediatrics & Neonatology
About a Case: Mass in the Colon
Jenny Arboleda1*, Alejandro Chiriboga1 and Edgar Vasconez2
1Cirujano Pediatra del Hospital Metropolitano, Ecuador
2Gastroenterologo del Hospital Metropolitano, Ecuador
*Corresponding author: Jenny Arboleda, Cirujano Pediatra del Hospital Metropolitano, Ecuador, India, Email: elizabethab2011@gmail.com
Submission: September 21, 2017; Published: January 22, 2018

ISSN : 2576-9200Volume1 Issue4
Introduction
Lynch syndrome is the most common cause of hereditary colorectal cancer (CRC). It is characterized by a significantly increased risk of CRC and endometrial cancer, as well as a risk of several other malignancies. Lynch syndrome is an auto somal dominant disorder that is caused by a germline mutation in one of several DNA mismatch repair (MMR) genes or loss of MSH2 expression due to deletion in the EPCAM gene (previously called TACSTD1) [1]. Among individuals with identifiable germ mutations in MMR genes, mutations in MLH1, MSH2, MSH6 and PMS2 are found in approximately 32, 39, 15 and 1, their sebut is in the over 30's [1].
Peutz-Jeghers syndrome (PJS), also known as hereditary intestinal polyposis syndrome, is an autosomal dominant disorder characterized by the development of benign hamartomatous polyps in the gastrointestinal tract and characteristic mucocutaneous pigmentations associated with a high risk of developing gastrointestinal and non-gastrointestinal malignancy [2-3]. The gene and mutation involved in the production of this pallium is located on chromosome 19p13.3, known as STK11 / LKB14- 5, and encodes a serine kinase threonine [4-6], with a possible suppressive action of tumor cells whose over expression induces to the growth of measured cells. Mutation of this gene increases the risk of developing colon and non-gastrointestinal cancer [5-6]. Peutz-Jeghers syndrome is a rare condition associated with familial adenomatous polyposis, its frequency is 1 case per 60,000 to 1 case per 300,000 live births in industrialized countries. A similar frequency is described in all races, the average age is in adulthood 23-26 years, it's distribution is similar in both sexes [7-8].
The risk of dying from gastrointestinal cancer is 13 times higher than the general population in patients with PJS [9]. The risk of other tumors of reproductive organs, pancreas, and lungs is 9 times higher than the general population [10,11]. Peutz-Jeghers syndrome is characterized by hyper-pigmentary lesions in the skin and mucous membranes and gastrointestinal polyps, these spots are located in the perineum 94%, hands 74%, buccal mucosa 66%, and feet 62% [12,13]. The most common areas of the gastrointestinal tract are the small intestine, jejunum, and ileus (65-95%), but may also be present in the colon (60%), stomach (50%), clinical course characterized by polyp-induced obstruction and bleeding. [14,15]. Differential diagnosis Hamartomatous polyps of the small intestine may be associated with Cowden's syndrome, Bannayan-Riley Ruvalcaba syndrome (BRRS), and juvenile polyposis syndrome (JPS). BRRS is characterized by macrocephaly, lipomas, vascular abnormalities and developmental delay. Cowden's syndrome has cutaneous features and oral findings including trichilemomas, acral keratoses, and oral papillomas / facial papules. Pigmented spots in BRRS and Cowden's syndrome typically occur in the penis of the glans in males and not in the lips as seen in PJS. Unlike PJS, which is associated with germline mutations in the STK11 gene, Cowden syndrome and BRRS are associated with germline mutations in PTEN1 [16].
Mucocutaneous pigmentation may be associated with Laugier- Hunziker syndrome (LHS). LHS is an acquired, sporadic, benign disorder characterized by intraoral hyperpigmentation on the lips, hard and soft palate, and buccal mucosa. Approximately 60 percent of patients with HSL also have hyperpigmented longitudinal bands on the toenails and no associated nail dystrophy. Unlike pigmentation in PJS, which occurs in the first years of life, LHS lesions are progressively acquired in young or middle-aged adults16. In addition, LHS is not associated with hamartomatous gastrointestinal polyps or with a pathogenic mutation in the STK1116 gene.
Diagnostic criteria proposed by Giardello SPJ
1 Small intestine polyposis, with histopathological confirmation of gastrointestinal hamartomatous polyps
2 History of positive family history
3 Pigmented macula in skin and mucous membranes having two of the three criteria makes the diagnosis.
Among the laboratory studies we will have microcytic anemia due to microscopic blood loss. It could be detected in carcinoembryonic antigen (CEA) useful for monitoring in cancer cases. Imaging studies (rx and abdomen, echo of abdomen and tomography of abdomen). Colonoscopy is useful for taking biopsies at the time of diagnosis [16,17]. The definitive diagnosis of the type of polyp is determined by histopathology. Laparotomy surgery is performed according to cases from a polypectomy, repair of invaginations and bowel resections 17 Recommendations Table 1.
Table 1:
Presentation of the Case
Patient of 8 years old, resident in Quito product of the second pregnancy; parents without pathological antecedents of importance obtained by normal delivery without complications to term, receiving breastfeeding up to 6 months, does not report antecedents of importance, siblings without history. He entered the emergency service of the Metropolitan Hospital with colic abdominal pain, vomiting for 3 occasions, and bloody stools scarce of 72 hours of evolution. It is a patient with CF: 85 / min, FR: 24 / min, PA: 100/60 mmHg, pale mucosa with mucosal staining, symmetrical thorax, no murmurs, abdomen palpable mass on the left flank of 5x3 cm, peristalsis enlarged, 3-second capillary full tips, rectal touching glove outlet stained with scarcely bloody stools Rx of abdomen is performed reporting uneven levels, ultrasound reports scarce free fluids, and CT reports mass in descending colon. Biometrics with neutrophil leukocytosis, cretinin in normal ranges. You enter with presumptive diagnosis of Obstructive Abdomen [18].
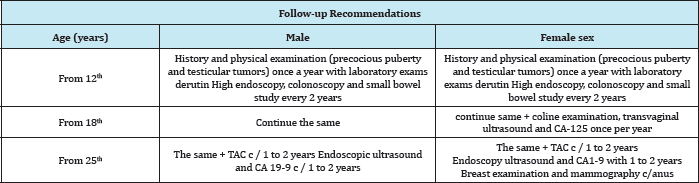
Entering surgery 03/03/2017 colonoscopy is performed (Figure 1) finding mass at the level of descending colon that obstructs 90% of light, continues with exploratory laparotomy finding the tumor mass at the level of descending colon that invaginaba the colon transverse, de-invagination, resection of the tumor mass (Figure 2) and end-colon-terminal anastomosis were performed. It is sent to pathology. Patient with good postoperative evolution, the result of pathology reports as suggestive of Lynch Syndromes, a genetic study is carried out in which Peutz-Jeghers de novo syndrome is reported, which is more compatible with the patient's clinic.
Figure 1: Colonoscopy, courtesy Dr Vasconez
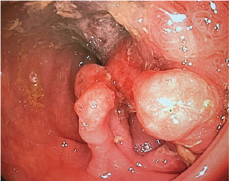
Figure 2: Transurgical, courtesy Dr Chiriboga.
Discussion
The presented case gave us to think oftwo colon mass syndromes such as Lynch syndrome and the result of genetics is quite specific? finding the typical mutation of the Peutz-Jeghers syndrome as are the characteristics exposed in the literature. The 8-year-old patient complies with two of the three criteria proposed by Giardello, the polyps in the colon 60% and the mucosal stains, lacking only the family history that is corroborated by the genetic study that indicates a de novo mutation, which also the brother performs the genetic study which is negative. The clinic is characteristic of the typical picture of intestinal obstruction in older children, with digestive bleeding that leads us to think of an imagination, which would lead us to think of other causes of imagination. The imaging and laboratory results also corroborated the suspicions that the biopsy shows us another diagnosis of similar histo pathological behavior, so we decided to perform the genetic study that indicates a mutation in the variant gene in STK11, consisting of the syndrome Peutz-Jeghers. Cancer risk includes 45-54% of breast cancer, 39% of colon cancer, 11-36 risk of pancreatic cancer, and a 29% risk of stomach cancer. Despite the scientific evidence, it is concluded that the SPJ is not frequent or is not easily recognized at the moment of the consultation, the management of the presented case was adequate after the opportune diagnosis due to the high degree of suspicion of the Hospital Pediatric Surgery service Metropolitan and working together with pediatric gastroenterology. In spite of the absence of familiar antecedents which they were searched exahustivamente. As for the prognosis of the disease, interesting studies revealed that the risk of cancer is 45-54% of breast cancer, 39% of colon cancer, 11-36 risk of pancreatic cancer, and 29% of cancer risk of stomach. Most patients with JPS develop some form of neoplasm by age 60. Although some hamartomatous polyps alone do not have malignant potential, they require continuous monitoring.
References
- Palomaki GE, McClain MR, Melillo S (2009) EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med 11(1): 42-65.
- Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, et al. (2006) Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 12(10): 3209-15.
- Marlene Anaya, AleAlejandra López (2010) Síndrome de Peutz-Jeghers, apropósito de un caso.. Gaceta medica Boliviana version.
- Jeghers H, McKusick VA, Katz KH (1949) Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J 241(26): 1031-1036.
- Peutz JL (1921) Over een zeer merkwaardige, gecombineerde familiaire pollyposis van de sligmliezen van den tractus intestinalis met die van de neuskeelholte en gepaard met eigenaardige pigmentaties van huid-en slijmvliezen. Ned Maandschr v Gen 10: 134.
- Jeghers H, McKusick VA, Katz KH (1949) Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med 241(25):993.
- Harned RK, Buck JL, Sobin LH (1995) The hamartomatous polyposis syndromes: clinical and radiologic features. AJR Am J Roentgenol 164(3): 565.
- Lindor NM, Greene MH (1998) The concise handbook of family cancer syndromes. Mayo Familial Cancer Program. J Natl Cancer Inst 90(14): 1039-1071.
- Olschwang S, Markie D, Seal S, Neale K, Phillips R, et al. (1998) Peutz- Jeghers disease: most, but not all, families are compatible with linkage to 19p13.3. J Med Genet 35(1): 42-44.
- Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, et al. (1998) Peutz- Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 18(1): 38-43.
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, et al. (1998) A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391(6663):184-187.
- Lim W, Hearle N, Shah B, Murday V, Hodgson SV, et al. (2003) Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer 89(2): 308-313.
- Boudeau J, Sapkota G, Alessi (2003) LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett 546(1): 159-165.
- Hernan I, Roig I, Martin B, Gamundi MJ, Martinez-Gimeno M, et al. (2004) De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet 66(1): 58-62.
- Baas AF, Kuipers J, Van der Wel NN, Batlle E, Koerten HK, et al. (2004) Complete polarization of single intestinal epithelial cells upon activation of LKB1 by Strad. Cell 116(3): 457-466.
- Martin SG, St Johnston D (2003) A role for Drosophila LKB1 in anterior- posterior axis formation and epithelial polarity. Nature 421(6921): 379384.
- Jansen M, de Leng WW, Baas AF, Myoshi H, Mathus-Vliegen L, et al. (2006) Mucosal prolapse in the pathogenesis of Peutz-Jeghers polyposis. Gut 55(1): 1-5.
- Volikos E, Robinson J, Aittomaki K, Mecklin JP, Jarvinen H, et al. (2006) LKB1 exonic and whole gene deletions are a common cause of Peutz- Jeghers syndrome. J Med Genet 43(5): e18.
© 2018 Jenny Arboleda, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






