- Submissions

Full Text
Research in Medical & Engineering Sciences
Characterization of Neutron Capture by Boron at Low Energies: Preliminary LET Results from a Geant4 Simulation
Robinson Steven Medina1#, Diego Alexander Tellez1#, Edwin Munévar2# and J Alfonso Leyva3*#
1Maestría en Ingeniería, Universidad Distrital Francisco José de Caldas, Colombia
2Proyecto Curricular de Licenciatura en Física, Universidad Distrital Francisco José de Caldas, Colombia
3Departamento de Física, Facultad de Ciencias, Pontificia Universidad Javeriana, Colombia
#All authors contributed equally to this work
*Corresponding author:J Alfonso Leyva, Departamento de Física, Facultad de Ciencias, Pontificia Universidad Javeriana, Cra. 7 No 40-62, Bogotá, 110231, Cundinamarca, Colombia
Submission: November 14, 2023;Published: November 29, 2023

ISSN: 2576-8816Volume10 Issue4
Abstract
Thermal neutron capture by 10B is a nuclear process with significant applications mainly in basic sciences, industry and medicine. This nuclear reaction generates α particles and 7Li ions, both with high Linear Energy Transfer (LET). These particles have an average displacement of the same order of magnitude of the eukaryotic cell diameter, which allows them to induce various biological effects inside the cell. To achieve a complete characterization of the boron neutron capture reaction, a Monte Carlo simulation was implemented via the Toolkit Geant4. The Linear Energy Transfer (LET) was calculated in this simulation, with the incident neutron beam energy set at 0.025eV. As the target, a water phantom with a 0.1% concentration of 10B was utilized. According to the results obtained, the α particles reach peak LET values close to 250keV/μm, while for 7Li ions, LET peak values of around 400keV/μm are found.
Keywords:Neutron radiation; Neutron capture therapy; Boron; Geant4; Medical Physics
Introduction
The discovery of the neutron [1] significantly advanced nuclear physics research, with far-reaching implications for medical applications, particularly in the realm of cancer therapy [2,3]. One notable outcome of this progress was the development of neutron capture therapy (NCT).
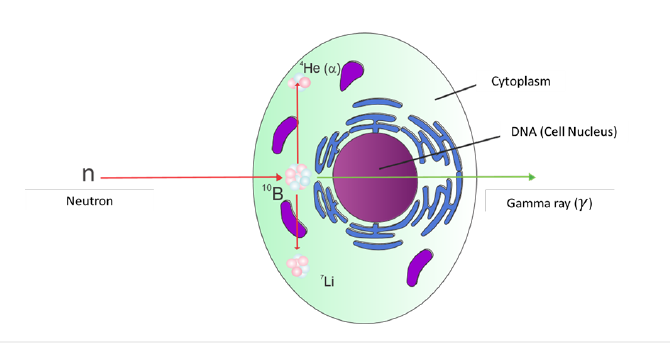
NCT introduces, at the cellular level, a marker agent, typically a neutron absorber such as 7Li, 10B, or 157Gd. These elements are strategically chosen for their ability to efficiently capture thermal or epithermal neutrons, a crucial step in the therapeutic process. This targeted approach allows for the production of localized ionizing radiation, offering a means to selectively damage cancerous tissue while minimizing adverse effects on healthy cells.
When boron (10B) is specifically employed as the absorbing medium in NCT, the technique is referred to as boron neutron capture therapy (BNCT). The corresponding reaction, schematically illustrated within the cell, is shown in Figure 1 [4]. The nuclear reaction
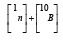
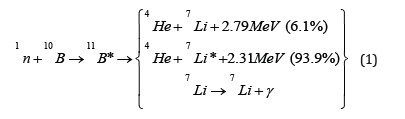
Figure 1:Scheme of the boron neutron capture reaction as it occurs inside the cell [4]. The final state includes 4He(α), 7Li, and, in most of the cases, γ radiation.
In this scenario, an excited
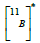
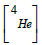
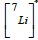
Given the potential of the particles generated in NCT to cause damage to cells, it is relevant to determine their angular, momentum, and kinetic energy distributions as discussed in [7]. This enables the kinematical characterization of the reaction, allowing for the simulation (for comparison purposes) of the interaction of each primary particle individually within a specific absorbing medium. Also, the dose distributions, as presented in [8], provide a means to explore the relationship between boron concentration, dose, and the effective distance range for the treatment of tumors. Additionally, understanding Linear Energy Transfer (LET) distributions is of paramount importance. These not only help us assess the potential radiation effects but also provide advantages in treatment planning and, eventually, treatment optimization. This significance is particularly pronounced in the context of heavy particle therapies involving α particles and 7Li ions. In this work, we address this need by presenting preliminary results obtained for the corresponding LET distributions as a function of depth within the target. We achieve this through a Geant4 [9] simulation, focusing on the primary decay products involved in BNCT.
Simulation
According to the ICRU report [10], LET is defined as the average energy lost by charged particles due to electronic interactions that occur when they traverse a distance dl in a specific material, minus the sum of the kinetic energy of secondary electrons exceeding a certain threshold value. For the purpose of studying radiobiological effects, it is necessary to resort to LET-dose (LETd), which is defined as the LET of each particle weighted in relation to the local dose. Numerically, LETd is expressed as:
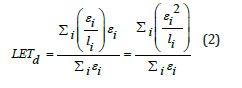
Where εi is the energy deposited by the primary particle in its i-th step of length li. The index i spans all the steps of the primary particle’s type across all events.
To determine the LET-dose associated with BNCT products, we developed a Geant4 simulation (version 10.7) using a cubical thin water target of dimensions (10×10×0.1)mm, containing a 0.1% concentration of 10B. The neutron source consists of a monoenergetic and monodirectional neutron beam with an energy of 0.025eV. We executed a total of 107 events, incorporating the G4EmStandardPhysics and QGSP BIC HP physics packages to handle electromagnetic and hadronic processes, respectively. Additionally, we included the G4NeutronHPThermalScattering package to account for thermal elastic scattering effects, which are dominant at low neutron energies. The LET-dose was calculated on an event-by-event basis using equation (2).
Result
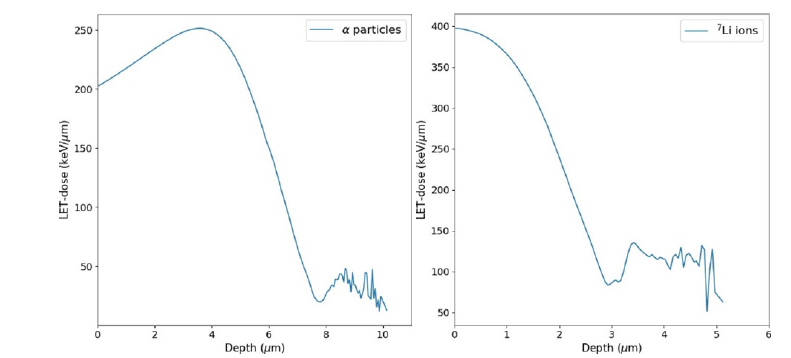
Figure 2 shows the LET-dose simulation distributions for the main BNCT decay channel obtained for α (1.47MeV) particles and 7Li (0.84MeV) ions using a low-energy neutron beam (0.025eV). Notably, the average displacement covered by these does not extend beyond 8μm for α and 3μm for 7Li, unequivocally indicating that their destructive effects are primarily confined within the diameter of a cell. Beyond these values, there are some fluctuations that can be attributed to low statistical accuracy. The maximum LET dose value achieved for α particles is approximately 250keV/μm, while for 7Li ions, it approaches 400keV/μm. The LET-dose distribution for α exhibits a discernible Bragg-peak structure at approximately 3.5μm, which is not observed for 7Li ions.
Supplementary Figure 2:LET-dose (LETd) simulation distribution for the main BNCT decay channel, as a function of depth for α particles (left) and for 7Li ions (right). The distributions reveal maximum LETd values of 250keV/μm and 400keV/ μm for α and 7Li, respectively. Additionally, it is evident that the displacement ranges for both of them are clearly contained within the diameter of a cell.
Concluding Remark
The primary focus of Boron Neutron Capture Therapy (BNCT) development is brain cancer, specifically glioblastoma multiforme (GBM), renowned for its aggressive nature and limited surgical removal [11]. This highly proliferative tumor extensively invades healthy tissue before symptom onset, resulting in a higher sterilization or destruction rate compared to conventional techniques like X-ray radiotherapy. The success of BNCT in treating such tumors relies on factors such as achieving an adequate 10B concentration in tumor cells, differential boron uptake, and substantial thermal neutron fluence in the tumor region [12]. Ongoing research is directed towards creating medications capable of differentially transporting boron to cancerous tissues and developing neutron sources that pose no threat to medical facilities. The exploration of compact neutron generators is currently a focal point of considerable interest.
In this context, the determination of Linear Energy Transfer (LET) is key to understanding the BNCT reaction, as it plays a crucial role in assessing the efficacy of the treatment. Our results show that α particles and 7Li ions can penetrate up to 8μm and 3μm, respectively, for a 10B concentration of 0.1%. These results are very promising, as the emitted products from the nuclear reaction, following neutron capture by Boron, have enough energy to generate damage to the cell. However, additional studies are necessary, such as the relative biological effectiveness (RBE), which enables a more realistic comparison between the BNCT and the standard X-ray treatment.
Acknowledgment
The computational work was conducted at the Computational Centers of Pontificia Universidad Javeriana and Universidad Distrital Francisco José de Caldas. The authors are grateful for the support of Pontificia Universidad Javeriana, Bogotá D.C., Colombia and Universidad Distrital Francisco José de Caldas, Bogotá D.C., Colombia, respectively.
Declarations
Funding: This study was funded by the internal budgets of the Faculty of Sciences of the Pontificia Universidad Javeriana and the Faculty of Sciences and Education of the Universidad Distrital Francisco José de Caldas.
Conflict of interest: The authors declare no competing interests.
Ethics approval: This article does not contain any studies on animals performed by any of the authors.
Consent for publication: Not applicable.
Availability of data: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability: The code associated to this study is available from the corresponding author upon reasonable request.
Authors’ contributions: Robinson Steven Medina, Diego Alexander Tellez, Edwin Munévar, and José Alfonso Leyva contributed equally to this work.
References
- Chadwick J (1932) The existence of a neutron. Proc R Soc Lond A 136(832): 692-708.
- Taylor HJ, Goldhaber M (1935) Detection of nuclear disintegration in a photographic emulsion. Nature 135: 341.
- Locher GL (1936) Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol 36: 1-13.
- Sauerwein WAG, Moss R, Wittig A, Nakagawa Y (2012) Neutron capture therapy principles and applications. Springer, Berlin, Germany.
- Puerta A, Morales J (2020) Biological effects of ionising radiation. Rev Colomb Cardiol 27(S1): 61-71.
- Barth R, Zhang Z, Liu T (2018) A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Communications (London) 38(1): 36.
- Medina RS, Téllez DA, Munévar E, Leyva JA (2018) Characterization of the nuclear reaction of boron neutron capture therapy (BNCT) by Geant4. Rev Investig Apl Nucl 2: 43-54.
- Leyva JA, Munévar E (2023) Current status and development of neutron radiation for biophysical applications in Colombia. Biophys Rev 15: 531-538.
- Agostinelli S, Allison J, Amako K, Apostolakis J, Araujo H, et al. (2003) Geant4-a simulation toolkit. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 506(3): 250-303.
- Edwards C (1999) Fundamental quantities and units for ionizing radiation -ICRU report 60. 21.
- Kankaanranta L, Seppala T, Koivunoro H, Saarilahti K, Atula T, et al. (2012) Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: Final analysis of a phase I/II Trial. Int J Radiat Oncol Biol Phys 82(1): e67-75.
- Moghaddasi L, Bezak E (2017) Development of an integrated Monte Carlo model for glioblastoma multiforme treated with boron neutron capture therapy. Scientific Reports 7(1): 7069.
© 2023 J Alfonso Leyva. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






