- Submissions

Full Text
Research in Medical & Engineering Sciences
Nontoxic Flame Retardants Based on Metal Oxide Nanoparticles
Anmar A Harhoosh*
Iraqi Ministry of Education, Baghdad, Iraq
*Corresponding author:Anmar A Harhoosh, Iraqi Ministry of Education, Baghdad, Iraq
Submission: August 22, 2023;Published: November 03, 2023

ISSN: 2576-8816Volume10 Issue4
Abstract
This review provides an overview of the health hazards of the polymeric materials emissions under combustion. As well as, addressing the importance of using metal oxides nanoparticles as flame retardants additives, especially ZnO, Fe2O3, MgO and Al2O3, since these nano-oxides catalyze the formation of thermal barrier (char barrier) to prevent polymeric materials to burn, and to prevent its toxic hazard emissions.
Keywords:Metal oxide nanoparticles; Toxic hazard emissions; Flame retardant
Introduction
The combustion of polymeric materials leads to toxic gases emission and products, like chlorine, styrene, and carbon monoxide CO. Styrene poses the greatest toxicological risk.
Styrene gas and carbon monoxide that emitted from polymeric materials during combustion leads to suffocation that goes to death, there are potential concerns of inhalation of such gases and products lead to health problems and increase in the probability of human cancer [1,2].
Figure 1:SEM photos of the char residues.
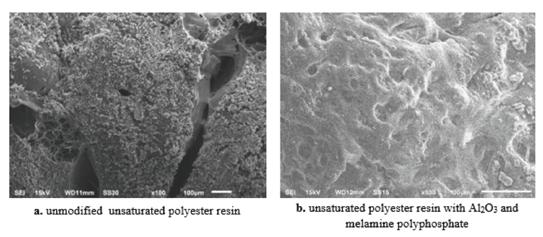
Thus, there’s an argument needs to use metal oxides nanoparticles as flame retardant additives, as they are going to make thermal barrier that prevents the rapid thermal deterioration of polymeric materials and thus lead to reduces the possibility of emitting toxic polymeric products when it faces to heat and fire [3,4]. The review focuses on the metal oxide nanoparticle as flame retardants additives to polymeric materials to enhance thermal properties of these materials, which lead to decrease the emission of toxic product. Many researchers used ZnO, Fe2O3, MgO and Al2O3 nanoparticles as flame retardants. Because of these metal oxides nanoparticles primarily contribute to the formation of additional double bonds and cross-links in the polymer molecules, which this process going to slow down the polymer degradation, by catalyzing the formation of char during combustion [5-8]. Therefore.
an increase in the char catalysis leads to the formation of char barrier that prevents the degradation of polymer and reduces its toxic emissions. Harhoosh et al. [9] presented the morphology of the carbonized residue sample that contains 6 mas. % of melamine polyphosphate and 1 mas. % of Al2O3 nanoparticles as shown in Figure 1. The presented morphologies were carbonized residue of the obtained samples after heated to 350 °C, they showed that the unmodified unsaturated polyester resin sample was cracked under heating Figure 1a, while the modified sample that contain 6 mas. % of melamine polyphosphate and 1 mas. % of Al2O3 nanoparticles was formed a carbonized layer or a ceramic layer on the surface of unsaturated polyester resin as shown in Figure 1b. In work [9] was investigated that the carbonized layer protects the substrate of unsaturated polyester resin material.
As mentioned above, it can be concluded that the using of metal oxides nanoparticles as flame retardants additives in the polymeric materials’ construction leads to decrease in the toxic effect of polymeric materials, by form a char barrier (carbonized layer) on the surface of polymers.
Conclusion
The idea of this review is to emphasize the need of metal oxides nanoparticles as flame retardants additives in polymeric materials to minimize the toxic effect of the burning polymeric materials hazard on our health and life. As well as the concept of char barrier that catalyzed to form by the metal oxides nanoparticles has been described.
References
- Banton MI, Bus JS, Collins JJ, Delzell E, Gelbke HP, et al. (2019) Evaluation of potential health effects associated with occupational and environmental exposure to styrene-an update. J Toxicol Environ Health B Crit Rev 22(1-4): 1-130.
- Alarie Y (1985) The toxicity of smoke from polymeric materials during thermal decomposition. Annual Review of Pharmacology and Toxicology 25: 325-347.
- Yurtov E, Harhoosh A (2020) Inorganic flame retardants to preserve human life and health. 2nd Scientific-practical conference of Russian and Croatian scientists in Dubrovnik. Moscow, Russia, pp. 61-62.
- Harhoosh A, Yurtov E, Bakhareva N (2022) Flame retardant strategies and the physical barrier effect of nanoparticles to improve the thermal performance of a polymer. Theoretical Foundations of Chemical Engineering 56: 545-553.
- Sertsova A, Marakulin S, Yurtov E (2017) Metal compound nanoparticles: Flame retardants for polymer composites. Russian Journal of General Chemistry 87: 1395-1402.
- Laachachi A, Leroyb E, Cocheza M, Ferriola M, Lopez Cuesta JM (2005) Use of oxide nanoparticles and organoclays to improve thermal stability and fire retardancy of poly(methyl methacrylate). Polymer Degradation and Stability 89(2): 344-352.
- Guoxin L, Junfen Y, Tingshu H, Yonghua W, Guozheng L (2008) An Investigation of the thermal degradation of the intumescent coating containing MoO3 and Fe2O3. Surface and Coatings Technology 202(13): 3121- 3128.
- Yuezhan F, Chengen H, Yingfeng W, Xingping Z, Xie X, et al. (2018) Multi-functional interface tailoring for enhancing thermal conductivity, flame retardancy and dynamic mechanical property of epoxy/Al2O3 Composites Science and Technology 160: 42-49.
- Harhoosh A, Yurtov Y (2021) Al2O3 nanoparticles preparation. And its application as flame retardants with melamine polyphosphate additives in composite materials based on unsaturated polyester resins. AIP Conference Proceedings 2372(1): 130027.
© 2023 Anmar A Harhoosh. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






