- Submissions

Full Text
Research in Medical & Engineering Sciences
Development of Eco-Friendly Carboxymethyl Cellulose Hydrogel from Sugarcane Bagasse
Md. Hasibul Islam1, Md. Monirul Islam1, Firoz Ahmed1,2, Siddhartha Sankar Saha1, Md. Shamsul Alam1, Md. Ahsan Habib1 and Md. Ibrahim H Mondal1*
1Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, University of Rajshahi, Bangladesh
2BCSIR Laboratories Rajshahi, Bangladesh Council of Scientific and Industrial Research, Bangladesh
*Corresponding author:Md. Ibrahim H Mondal, Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, University of Rajshahi, Rajshahi-6205, Bangladesh
Submission: March 13, 2023Published: May 31, 2023

ISSN: 2576-8816Volume10 Issue3
Abstract
A carboxymethyl cellulose-g-(methyl methacrylate-co-acrylamide) was prepared from Sugarcane bagasse. The physical properties (e.g., degree of swelling, water desorption and gel content) of hydrogels were determined. FTIR analysis showed that the characteristic absorption band of MMA and AAM repeating units appeared in the spectra of the product and that confirmed the grafting of MMA and AAM monomers onto the CMC backbone. The maximum water absorption capacity of the prepared hydrogels was 50g/g in deionized water. The surface morphology of newly synthesized hydrogels was also identified by SEM. TGA, XRD, biodegradable properties and metal ions uptake of hydrogels were also investigated. The result ensures that the prepared hydrogel can be effectively used in personal absorbent products, biomedical applications, and polluted water treatment.
Keywords:Sugarcane bagasse; Carboxymethyl cellulose; Biodegradable; Hydrogel
Introduction
Cellulose is the most abundant naturally occurring polymer obtained from plants and vegetable sources, including wood, jute, flax, bamboo, cotton, wheat straw, and sugarcane bagasse. It is a biodegradable, renewable, and ecologically beneficial polymer. Sugarcane bagasse, a byproduct of the Sugar Industry, is a possible source of cellulose. It has been observed that sugarcane bagasse comprises 40-50% cellulose, 25-35% hemicelluloses, and 18-24% lignin [1]. Hydrogels are cross-linked 3D polymeric networks which have the ability to absorb large amounts of water or biological fluids in their structures due to the presence of -NH2, -COOH, -OH, -CONH2, -CONH-, -SO3H groups [2]. Carboxymethyl cellulose (CMC) provides an extensive survey on cellulose and is also obtained from the agro cellulosic waste after the cultivation of sugarcane residue or byproduct of the sugar industry. Hydrogels from cellulose are three-dimensional, hydrophilic and also generally highly biocompatible, polymeric networks which are capable of absorbing large amounts of water or biological fluids. Due to their high-water content, porosity and soft consistency, they closely simulate natural living tissue, more than any other class of synthetic biomaterials [3]. However, degradation is not always desirable depending on the time frame and location of the drug delivery device [4].
The first crosslinked network material poly-hydroxy- ethyl methacrylate (PHEMA) hydrogel developed much later, in 1960, with the ambitious goal of using them in permanent contact applications with human tissues. Hydrogels are in fact the first materials developed for use inside the patient [5,6]. Since then, the number of studies about hydrogels for biomedical applications began to rise, especially in the decade of 70s [7]. Since the work of Wichterle and Lim in 1960 on cross-linked hydrogels, these hydrogels have been of great interest to biomaterial scientists for many years because of their hydrophilic and biocompatible character [8]. The aim of this study was to develop sugarcane bagasse (SBC)- based biodegradable superabsorbent hydrogels (SAH) with good swelling.
Material and Methods
Material
The basic material used for the research was sugarcane bagasse which was obtained from Rajshahi Sugar mills in Bangladesh. All chemicals and solvents used for this investigation were of reagent grade.
Preparation of cellulose from sugarcane bagasse: The extractive-free bagasse (40g) was first treated with distilled water for 2h at 70 and 80 °C respectively. Further, the two water-soluble free samples were de-lignified with 1% sodium chlorite at pH 3.5- 4.0 and adjusted with 10% acetic acid at 75 °C for 2h. Finally, the holo-cellulose was extracted with 17.5% sodium hydroxide for 10h at 50 °C respectively. After filtration, the two residues were washed thoroughly with distilled water and 95% ethanol and dried in an oven for 16h at 60 °C [9].
Preparation of carboxymethyl cellulose: About 5.0g of sugarcane bagasse was weighed and added to 250mL of distilled water. Then, 10mL of 30% (w/v) sodium hydroxide was added drop-wise and stirred for an hour. The carboxymethylation reaction was started by adding 6.0g sodium mono-chloroacetate with a reaction mixture placed in a thermo-stated water bath and heated up to 50 for 3h. The mixture was then filtered and the residue was suspended in 300mL of methanol overnight. The suspended methanol solution was then neutralized using glacial acetic acid. The residue was filtered and dried for constant weight [10].
Preparation of hydrogel: 0.3g carboxymethyl cellulose was dispersed in 20ml of distilled water in a 250ml four-necked flask and then 0.03g potassium per-sulfate was added and stirred for 10 min at 70 0C in a water bath. Then 0.3g methyl-methacrylate, 0.3g acrylamide and 0.03g N, N-methylene bis-acrylamide were added and stirred for 30 min at 70 in the water bath. Then the treatment continued for 2.5h. At the end of the reaction, the prepared hydrogels were removed carefully and washed with distilled water for several days. Again, the hydrogels were washed several times with pure ethanol for dewatering and immersed in NaOH solution for hydrolysis for 24h. Finally, the hydrogel was washed with distilled water and dried in an oven. The product was called carboxymethyl cellulose-g-(methyl methacrylate-co-acrylamide) [11]..
Gel content of hydrogel: The gel fraction of hydrogels was evaluated by immersing the sample in distilled water at room temperature and measuring the insoluble part of the sample. Using water to extract the soluble or unreacted parts of the samples, they were then removed and dried in a vacuum oven at 80 °C temperature to achieve a constant weight.
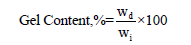
Where, Wd is the weight of the dried hydrogel after extraction, and Wi is the initial weight of the dried hydrogel [12].
Swelling behavior and water desorption of hydrogel: A dried sample (1cm×1cm) was immersed in distilled water at 25 °C and withdrawn at predefined time intervals. Surface water is extracted using filter paper. Swollen hydrogels were weighed and the percentage of water uptake capacity was measured by the equation below.
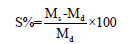
where S is the equilibrium water absorbency (%), Ms and Md are the weight of swollen hydrogel (g) at the time (t), and dry hydrogel (g), respectively [13].
The prepared hydrogel samples were weighted initially and then placed in an open environment for water desorption. At every 4h interval, the weight of the samples was taken for 0, 4, 8, 12 and 16 hours and it was continued for 24, 48, 72 and 96 hours until constant weights were established. Finally, the percentage of water desorption was calculated gravimetrically at room temperature. Water desorption of the hydrogel can be calculated as
Water desorption, Wd = [(Wo- Wt)/Wo]×100
Where Wt is the weight of the dry hydrogel after air drying time and Wo is the weight of the prepared hydrogel.
FTIR analysis
ATR-FTIR of the samples was conducted by an FTIR spectroscope (Model: FTIR-8900, Shimadju, Japan) for the observation of functional groups present in samples within the frequency range from 400 to 4000 cm-1 by the method of transmission [12].
TGA analysis
Thermal analyses of all samples were carried out with Perkin- Elmer Simultaneous Thermal Analyzer (STA 8000, Netherlands). The tests were carried out between 30° to 700 ℃ in an inert nitrogen atmosphere. The heating rate and the airflow rate were 20 ℃/min and 200ml/min, respectively.
SEM analysis
The surface morphology of the prepared hydrogels was observed by scanning electron micrograph (Hitachi, Model-S3400 N, VP SEM, Japan).
XRD analysisThe crystallinity of CMC and hydrogel were investigated by a PAN Analytical X Pert PRO X-ray diffractometer (Model: Rigaku, SmartLab, Japan). The scanning rate was 2/min over a period with a scan angle from 5°-70°.
The crystallinity of CMC and hydrogel were investigated by a PAN Analytical X Pert PRO X-ray diffractometer (Model: Rigaku, SmartLab, Japan). The scanning rate was 2/min over a period with a scan angle from 5°-70°.
Biodegradation study
The biodegradability of the samples was assessed by the soil burial method. Conventional garden soil (30 ℃, pH 6.0-8.5) was filled in plastic containers and moisture content was maintained at around 60%. The following equation was used to calculate the per cent of biodegradation- Rate of Biodegradation
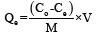
Where Wd is the dried weight of the hydrogel. Wi is the initial weight of the hydrogel; RB is the rate of biodegradation of the sample. [14].
Adsorption of metal ions
The amount of adsorbed metal ions was calculated by immersing a certain amount of synthesized non-hydrolyzed hydrogels in separate solutions (100ml) containing 200ppm of Cu2+ and Cr3+ with stirring at 100rpm for 3h. After filtration, the concentration of the filtrate was determined by Atomic Absorption Spectrometry (AAS). The amount of metal ions adsorbed by the hydrogel was expressed by the following equation-
where Co and Ce are the initial and final concentrations of the aqueous metal solution, respectively, V is the volume of metal and M is the weight of dried hydrogel sample. The outcome is resented as “metal ion removal percentage” in milligrams per gram of dry hydrogel sample [15].
Result and Discussion
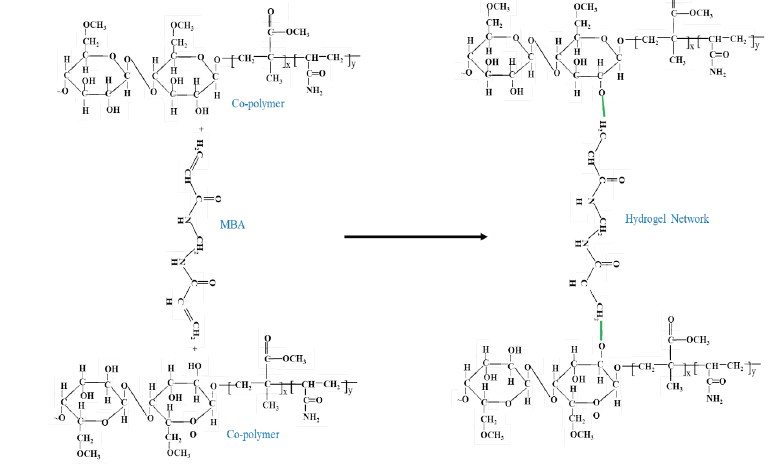
The reaction mechanism of carboxymethyl cellulose from sugarcane bagasse to prepare carboxymethyl cellulose-g- (methyl methacrylate-co-acrylamide) hydrogel is shown below.
Step 1: Carboxymethyl cellulose reacts with methyl-methacrylate and acrylamide in presence of potassium persulfate to form the copolymer network.
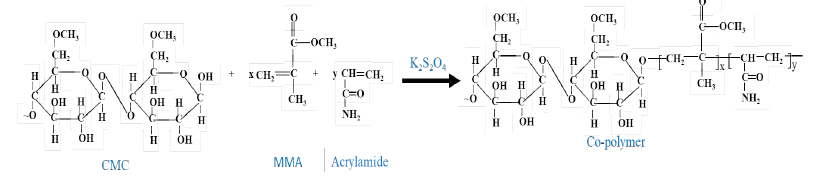
Step 2: Finally, the co-polymer reacts with N, N-methylene bis-acrylamide as a cross-linking agent to form the three-dimensional structure of hydrogel.
Gel content
Gel content indicates the degree of crosslinking formed in the hydrogel. The average gel content% of the prepared hydrogel was 82%.
Swelling and water desorption behavior
Figure 1a showed that the swelling of hydrogel increased with time up to 119.19% and then decreased with increasing time. At first, due to having high capillary force, water entered the prepared hydrogel pores and then diffusion occurred and the hydrogel started swelling [16]. Then the pores are compressed and extra water is expelled from the inside of the hydrogel. This indicated the porosity of the prepared hydrogel was very high. The dependence of hydrogel swelling on temperature was represented in Figure 1b. Swelling increased up to 70 ℃ and then decreased with increasing temperature. This happened due to lower critical solution temperature (LCST). The lower critical solution temperature for the prepared hydrogel might be 70 ℃ and above the LCST and hydrophobic interaction among the molecules present in the hydrogel backbone became dominating resulting in collapsed hydrogels and the expelling of water. For this reason, the swelling decreased with increasing temperature above 70 ℃ [17], but below the LCST, the prepared hydrogel absorbed water naturally.
Figure 1:Effects on swelling of prepared hydrogel.
a) swelling vs time,
b) swelling vs temperature,
c) swelling vs crosslinker,
d) desorption vs time and
e) Absorption vs time.
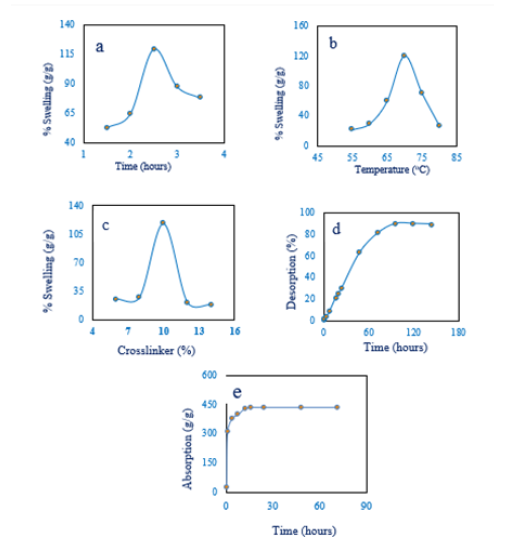
The maximum swelling was obtained at 10% cross-linker with respect to carboxymethyl cellulose (CMC) but minimum swelling was obtained at 14% cross-linker Figure 1c. Basically, the crosslinking agent creates a three-dimensional network among polymer chains with higher interstitial spaces. But excess loading of crosslinking agent occupies the interstices of the polymer network due to intermolecular interaction. Swelling increased with increasing the concentration of crosslinker but excess loading of crosslinking agent (above 10%) reduced the swelling process [18].
In Figure 1d, the water-swelled hydrogel was kept in the open air and water desorption was measured gravimetrically. From the figure, it was found that the de-sorption occurred linearly up to 100h and then reached equilibrium. After reaching equilibrium, the loss of mass rate remained constant with time. The absorption characteristics of water by the prepared hydrogel was represented in Figure 1e. The hydrogel was capable of absorbing water very quickly almost time-independent. From this experiment, it is clear that the prepared hydrogel was a good absorbent and could be used in medical dressing materials and separation techniques.
Fourier Transform Infrared (FTIR) analysis
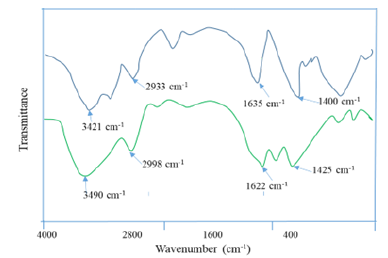
Figure 2 shows that the peak at 3421cm-1 and 2933cm-1 are identified as -OH stretching of carboxylic acids. and C-H stretching of Na-CMC. The peak at 1635cm-1 indicates C=O stretching vibrations due to the presence of carboxylic acids. The peak at 1400cm-1 could be assigned for -CH2- bending modes of Na-CMC. In Figure 2, the carboxymethyl cellulose-g-(methyl methacrylate-co-acrylamide) hydrogel, the peak at 3490cm-1 corresponds to the absorption of hydrogen bonds caused by O-H and the Peak at 1622cm-1 for N-H indicated stretching vibration of carboxamide functional group. The peak at 1425cm-1 was also assigned to the C-N bonding. These observations were logical to make the decision that the successful modification (grafting) had been done.
Figure 2:FTIR spectra of a) NA-CMC and b) Hydrogel.
TGA analysis
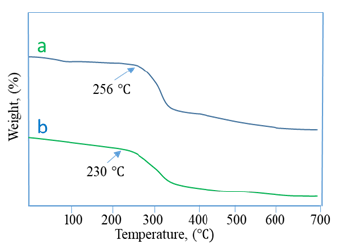
In Figure 3, the first step was seen up to 112 ℃ for both samples which was related to the loss of water from the samples. In the second step, the weight loss was due to the thermal decomposition of the polymeric chain. From this figure, it is clear that Na-CMC decomposition started at 256 ℃ but hydrogel started to decompose from 230 ℃. That is to say, the thermal stability of the prepared hydrogel is enough to use it for various applications.
Figure 3:TGA of a) NA-CMC and b) Hydrogel.
Scanning Electron Microscopy (SEM)
Figure 4 shows the morphology of the prepared hydrogel and Na-CMC obtained by SEM. Na-CMC had smooth and continuous fibre surfaces while hydrogels had rough, porous, and discontinuous surfaces. The porous surfaces of hydrogels help water diffusion in the polymeric network, thereby producing higher swelling capacity in the hydrogel.
Figure 4:SEM images of a) Hydrogel, and b) Na-CMC.

XRD analysis
Figure 5:XRD of a) NA-CMC, and b) Hydrogel.
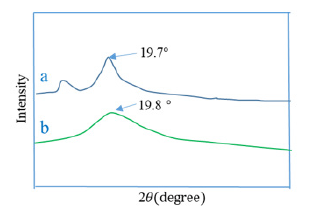
When comparing the X-ray diffraction patterns of Na-CMC and hydrogels from Figure 5, the characteristic peak at 19.7 for Na- CMC was obtained but the broad around at 19.8° with the smaller intensity of the prepared hydrogel was obtained. It confirmed that the prepared hydrogel has a lower degree of crystallinity with high elasticity.
Biodegradation study
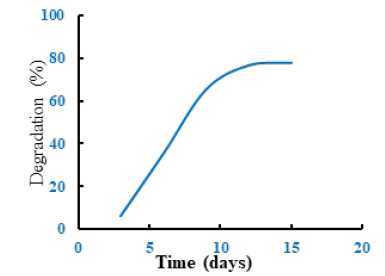
The bio-degradability study of the prepared hydrogel was carried out by the soil buried method. The biodegradability of hydrogel increased with time. It was seen that the minimum biodegradability of hydrogel was 6.25% at time 3 days and the maximum biodegradability of hydrogel was 78.13% after 15 days. The prepared hydrogel is mostly biodegradable in nature. Due to its high bio-degradability it’s eco-friendly (Figure 6).
Figure 6:Bio-degradability study of the prepared hydrogel.
Metal ions uptake
The absorption of metal ions by the hydrogel was 353mg/g and 255mg/g for Cr3+ and Cu2+ respectively. This is due to the difference in ionic radii of the ions. The smaller the ion size more chance it to enter into the pore of hydrogel easily.
Conclusion
In this study, carboxymethyl cellulose-based superabsorbent hydrogel was prepared from the waste sugarcane bagasse and their characterizations were investigated. Various physical properties were examined for superabsorbent hydrogel. Those properties improved the quality of carboxy-methylcellulose-g-(methyl methacrylate-co-acrylamide) superabsorbent hydrogel. Grafting of (methyl methacrylate-co-acrylamide) copolymer on the backbone of carboxymethyl cellulose and cross-linked three-dimensional network structure of hydrogels was confirmed by FTIR analysis. The morphology of synthesized hydrogel was confirmed by SEM. The result ensures that the prepared hydrogel can be used in personal care and biomedical applications as well as for the removal of metal ions from polluted water.
References
- Plermjai K, Boonyarattanakalin K, Mekprasart W, Pavasupree S, Phoohinkong W, et al. (2018) Extraction and characterization of nanocellulose from sugarcane bagasse by ball-milling-assisted acid hydrolysis. AIP Conference Proceedings 2010(1): 020005.
- Chai Q, Jiao Y, Yu X (2017) Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 3(1): 6.
- Caló E, Khutoryanskiy VV (2015) Biomedical applications of hydrogels: A review of patents and commercial products. European Polymer Journal 65: 252-267.
- Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: Progress and challenges. Polymer 49(8): 1993-2007.
- Lee SC, Kwon IK, Park K (2013) Hydrogels for delivery of bioactive agents: a historical perspective. Advanced Drug Delivery Reviews 65(1): 17-20.
- Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon LC, et al. (2021) Cellulose and its derivatives: Towards biomedical applications. Cellulose 28(4): 1893-1931.
- Seow WY, Hauser CAE (2014) Short to ultrashort peptide hydrogels for biomedical uses. Materials Today 17(8) 381-388.
- Kono H, Teshirogi T (2015) Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. International Journal of Biological Macromolecules 72: 299-308.
- Sun JX, Sun XF, Zhao H, Sun RC (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polymer Degradation and Stability 84(2): 331-339.
- Pushpamalar V, Langford SJ, Ahmad M, Lim YY (2006) Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydrate Polymers 64(2): 312-318.
- Marcì G, Mele G, Palmisano L, Pulito P, Sannino A (2006) Environmentally sustainable production of cellulose-based superabsorbent hydrogels. Green Chemistry 8(5): 439-444.
- Hossain M, Afroz S, Islam MU, Alam AKM, Khan RA et al. (2021) Synthesis and characterization of polyvinyl alcohol/water-hyacinth (Eichhornia crassipes) based hydrogel by applying gamma radiation. Journal of Polymer Research 28(5): 167.
- Seki Y, Altinisik A, Demircioğlu B, Tetik C (2014) Carboxymethylcellulose (CMC)-hydroxyethylcellulose (HEC) based hydrogels: synthesis and characterization. Cellulose 21(3).
- Xu Y, Hanna MA (2005) Preparation and properties of biodegradable foams from starch acetate and poly (tetramethylene adipate-co terephthalate). Carbohydrate Polymers 59(4): 52-529.
- Paulino AT, Guilherme MR, Reis AV, Campese GM, Muniz EC, et al. (2006) Removal of methylene blue dye from an aqueous media using superabsorbent hydrogel supported on modified polysaccharide. Journal of Colloid and Interface Science 301(1): 55-62.
- Chavda H, Patel C (2011) Effect of crosslinker concentration on characteristics of superporous hydrogel. International Journal of Pharmaceutical Investigation 1(1):17-21.
- Ehrenhofer A, Elstner M, Wallmersperger T (2018) Normalization of hydrogel swelling behavior for sensoric and actuatoric applications. Sensors and Actuators B: Chemical 255: 1343-1353.
- Budianto E, Muthoharoh SP, Nizardo NM (2015) Effect of crosslinking agents, pH and temperature on swelling behavior of cross-linked chitosan hydrogel. Asian Journal of Applied Sciences 3(5): 581-588.
© 2023 Md. Ibrahim H Mondal. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






