- Submissions

Full Text
Research in Medical & Engineering Sciences
Progress on the Preparation and Applications of Pentosan Sulfate
Ning Liu1,2, Fengqing Zhang1, Ye Liu2 and Long Zhang2*
1College of Chemistry and Life Science, Changchun University of Technology, Changchun, Jilin, 130012, P.R. China
2Jilin Provincial Engineering Laboratory for the Complex Utilization of Petro-resources and Biomass, School of Chemical Engineering, Changchun University of Technology, Changchun, Jilin, 130012, P.R. China
*Corresponding author: Long Zhang, Jilin Provincial Engineering Laboratory for the Complex Utilization of Petro-resources and Biomass, School of Chemical Engineering, Changchun University of Technology, 2055 Yanan Street, Changchun, Jilin, China
Submission: March 03, 2023Published: March 15, 2023

ISSN: 2576-8816Volume10 Issue2
Abstract
Heparin is a natural anticoagulant from animal organs with excellent anticoagulant activity, and it is of high price as the raw material for the preparation of heparin originates from the special organs of animals. There is also the problem of biological rejection. As Pentosan sulfate has a similar chemical structure to heparin and is prepared from renewable plant-derived pentosan, so the preparation and biological activity evaluation of pentosan sulfate has become a new research hotspot in the field. This paper mainly reviews the structure, physical and chemical properties, preparation methods and the analysis and evaluation of various biological activities of pentosan sulfate. The future development of pentosan sulfate is to find plant-based pentosans with similar structure to heparin, to develop the green technology for the controllable preparation and to explore the relationship between the bioactivity and its physical and chemical properties of pentosan sulfate to accelerate its practical application in medicine and biomaterials.
Keywords:Heparin; Pentosan; Pentosan sulfate; Biological activity; Green preparation; Application
Introduction to pentosan sulfate
Structure
Pentosan is composed of pentose, arabinose and xylose, and is one of the most abundant polysaccharides in nature. The chain of pentosan is mainly composed of repetitive five-carbon sugar units polymerized, including a small amount of phenolics and heteropolysaccharides. Pentosan sulfate is formed by modifying the hydroxyl group of pentosan and replacing the hydroxyl group with sulfate group, demonstrating multiple bioactivities. The mechanism of sulfation modification is the esterification of the hydroxyl group of the polysaccharide in solution by the sulfonic acid group in the sulfonating agent under the catalysis of Lewis acids, dehydration and then neutralization by alkali to obtain the sulfated polysaccharide [1] (Figures 1&2).
Figure 1:Structure diagram of pentosan dimer.
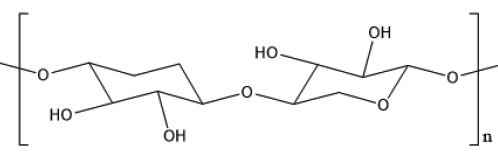
Figure 2:Simple formula of pentosan sulfate.
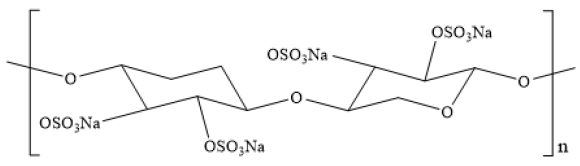
Physicochemical properties of pentosan sulfate
The physical properties of pentosan are mainly reflected in the
following aspects.
A. water-holding: pentosan can absorb more than 10 times
its own weight of water, so wheat bran pentosan can be used in
the food industry as a water-holding agent.
B. high viscosity characteristics: pentosan has a very high
viscosity. For example, in practical application, wheat bran
alkali-soluble pentosan can be used as a thickening agent.
C. emulsification stability: in practical applications, pentosan
with excellent emulsion stability can be considered as emulsion
stabilizer [2].
The chemical properties of pentosan are mainly:
a) Oxidative cross-linking: under the action of certain
chemical oxidants and oxidase systems, pentosan molecules
are cross-linked with each other to increase the viscosity
of the solution, which is the unique oxidative cross-linking
property, and water-soluble pentosan with better oxidative gel
characteristics can be used as a gel formation aid [3].
b) Catalytic degradation: pentosan can be degraded by the
action of xylanase, arabinase, hemi-cellulase, pentosanase and
chemical acid catalysts, etc. and the molecular size and structure
of pentosan can be changed, thus changing its properties [4].
c) Pentosan molecules contain phenolic acid structures
such as ferulic acid, which can form a conjugated structure
with hydrogen atoms and exhibit good antioxidant properties,
which can be used as natural antioxidants and used in food and
pharmaceutical industries [5].
d) Pentosan exhibits powerful antibacterial activity against
Gram-positive strains of bacteria [6].
Pentosan sulfate is a white odorless powder, slightly hygroscopic, soluble in sodium hydroxide solution, transparent, viscous at higher concentrations, insoluble in organic solvents such as ethanol, acetone, ether and n-butanol [7]. Sulfated pentosan also has higher water-holding capacity, higher viscosity and consistency compared to unmodified pentosan [8].
The chemical properties of Pentosan sulfate include:
A. Hydrogen peroxide and copper acetate can react with
pentosan sulfate to undergo oxidative depolymerization.
Hydrogen peroxide provides hydroxyl radicals, and free radicals
can induce degradation of pentosan sulfate to exert antioxidant
activity [9].
B. Enzymatic degradation: pentosan sulfate can be
depolymerized by hydrolysis and β-elimination mechanisms in
the presence of specific glycoside hydrolases and polysaccharide
lyases, respectively, and disrupting the glycosidic bond,
which acts synergistically by sulfate esterase to give neutral
monosaccharides and oligosaccharides [10].
Main uses
Products of natural origin, such as polysaccharides, have attained various applications in the fields of medicine, nutritional foods, medicinal cosmetics and functional foods. Studies have shown that natural polysaccharides modified by sulfation can not only enhance their original biological activities, but also generate new biological activities. Sulfated polysaccharides are non-toxic and can be used in foods, drugs, and health products [11,12].
Pentosan is the key non-starch polysaccharide in wheat seeds and is the main component of dietary fiber, which has the effect of lowering lipids and sugar and improving intestinal flora. Adding pentosan to bread-making flour can increase the water absorption capacity of flour and meet the level of dietary fiber for human consumption. Wheat bran pentosan has been applied in the pasta and beverage industry and is still in the research and development stage in meat and confectionery products, and also plays a definite role in animal feed and fruit and vegetable preservation. However, more top-end pentosan-derived products are needed further research and development such as pentosan sulfate, et al. [13,14].
Sulfated polysaccharide drugs include naturally derived glycosaminoglycans and semi-synthetic sulfate polysaccharides, etc. They mainly include heparin, chondroitin sulfate, fucosan sulfate, sodium alginate diester, and glycosyl ester. In recent years, sulfated polysaccharide drugs have been increasingly used in clinical applications, such as pentosan polysulfate (PPS), which is approved by the Food and Drug Administration and marketed in the United States for the treatment of interstitial cystitis [15,16]..
Algal polysaccharides can form hydrogels, including fucosan, alginate, carrangeenan and other polysaccharides with sulfate groups, all of which have unique structural and functional properties. Many of these materials have been shown to have skin-protective properties, including anti-wrinkle, whitening, moisturizing, UV protection, antioxidant and anti-inflammatory activities. This has expanded their uses in cosmeceuticals as emulsifiers, stabilizers and viscosity adjustment ingredients. Various polysaccharides in cosmetics functions moisturizing, stabilizing, skin tone improving, anti-aging, antibacterial and the polysaccharides are non-toxic and have good compatibility with commonly used cosmetic ingredients; therefore, bioactive polysaccharides will have a promising future as efficacious cosmetic additives [17,18].
Pentosan sulfate has been increasingly reported for its versatile biological activities, including anticoagulant activity, followed by antitumor, antiviral, anti-inflammatory and antioxidant activities. et al [19].
Progress in the Preparation of Pentosan Sulfate
Concentrated sulfuric acid sulfonation
Concentrated sulfuric acid is used as an esterifying agent to react with pentosan in n-butanol, and the sulfate group esterifies the hydroxyl group in the polysaccharide to produce sulfate ester, and then sodium hydroxide is added to neutralize the hydrogen ions. The reaction is as follows.
The concentrated sulfuric acid sulfonation was performed by adding n-butanol (2.5mL) to a three-necked flask with a pipette and slowly adding concentrated sulfuric acid (7.5mL) dropwise in an ice-water bath reacting for 1h and adding concentrated sulfuric acid (2.5mL) slowly in 0OC ice water bath continuing to stir until completely mixed. Slowly add 1.0g of pentosan powder and react for 6h at 0 ˚C under continuous stirring. After the reaction is completed, slowly add NaOH solution (5mol/L) and adjust to pH=7, then slowly add three times the volume of anhydrous ethanol under stirring and settle at low temperature for 12h. The precipitate was obtained by filtration. The precipitate was added to 20mL of distilled water, stirred until it was completely dissolved and put it into a dialysis bag and dialyzed under running water for 72h. Finally, barium chloride solution was used to test whether the NaCl was removed. The dialysate was concentrated to 15-20mL and lyophilized. The final calculated yield is 23.91%. Currently, the degree of substitution of sulfated polysaccharide is 0.195 [20] (Figure 3).
Figure 3:Concentrated sulfuric acid esterification mechanism.
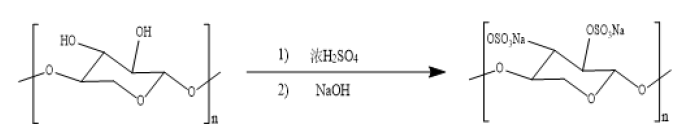
The concentrated sulfuric acid sulfonation is simple, low cost, but the yield, sulfate content and substitution degree of the obtained sulfate polysaccharides are low. Concentrated sulfuric acid easily causes polysaccharide degradation and serious pollution and corrosion of equipment, and the process parameter is difficult to control. It is important to further improve the product yield and alleviate excess concentrated sulfuric acid pollution.
Pyridine chloro-sulfate sulfonation
Figure 4:Reaction mechanism of pyridine chlorosulfate method.
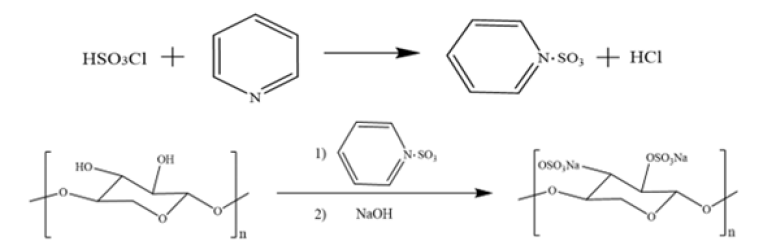
The addition of chlorosulfonic acid to anhydrous pyridine forms a chlorosulfonic acid-pyridine complex, followed by the addition of pentosan, which can be viewed as a monochloride of sulfuric acid and when the alcoholic hydroxyl group of the substance undergoes a substitution with chlorinicion and the corresponding sulfate product is produced [21]. The reaction mechanism is shown in the following Figure 4.
Preparation of esterification reagent: Take pre-chilled anhydrous pyridine (30mL) in a three necked bottle (100mL) with a drying tube, make a low temperature environment of -20 °C in a salt bath, and add 10mL of chlorosulfonic acid slowly dropwise with a constant pressure funnel under stirring (always control the temperature less than 5 °C during the reaction).
Preparation of the sulfate: After the end of esterification reagent preparation, transfer the three necked bottle to an oil bath and slowly increase the temperature until the turbid solid in the three necked bottle dissolves slowly. If insoluble, add some C5H5N or DMF to promote dissolution. After the esterification reagent is completely dissolved, slowly add 0.5g of pentosan powder in the oil bath under electric stirring and heat to 70 °C for 6h. After the reaction is completed, cool to room temperature and adjust the pH to neutral by adding NaOH solution (3mol/L) dropwise in an ice water bath. Slowly add three times the volume of anhydrous ethanol while stirring and settle at low temperature for 12h. The precipitate was obtained by filtration. The precipitate was added to 20mL of distilled water, stirred until it was completely dissolved, and put it into a dialysis bag and dialyzed under running water for 72h. Finally, barium chloride solution was used to test whether the NaCl was totally removed. The dialysate was concentrated to 15-20mL and lyophilized. The final calculated yield is 108.68%. At this time, the degree of substitution of sulfated polysaccharide is 1.276 [20]. Chlorosulfonic acid can be regarded as SO3·HCl complex, which is a common esterifying agent for the preparation of sulfated polysaccharides in a laboratory. The chlorosulfonic acid-pyridine method is more suitable for pyranose with simple procedure and cheap materials and high degree of substitution. However, chlorosulfonic acid is corrosive, the process rate of esterification is slower with many side reactions and toxic hydrogen chloride gas is produced and resulting negative environmental impact.
Sulfur trioxide pyridine sulfonation
Theoretically, sulfur trioxide is the most direct and effective esterifying agent as it contains -SO3H group that can be introduced directly into the reaction. Sulfur trioxide complex is usually prepared by passing sulfur trioxide into the corresponding organic base or by adding the organic base to a dispersion system of sulfur trioxide organic solvent. Sulfur trioxide is added to anhydrous pyridine to form sulfur trioxide-pyridine complex, and then pentosan is added and the -SO3 group esterifies the hydroxyl group in the polysaccharide to produce sulfate [21]. The reaction mechanism is shown in the following Figure 5.
Figure 5:Sulfur trioxide pyridine method reaction mechanism.
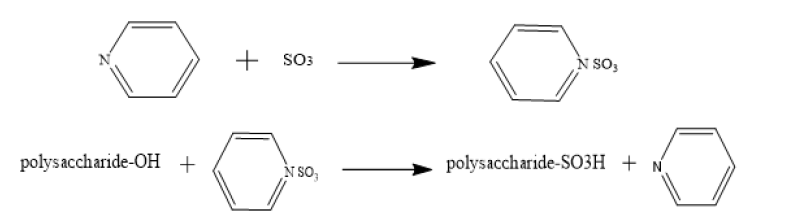
48mL pyridine (pentosan-pyridine ratio 1:60) was added to 100mL three- mouth bottle (with condensing tube, drying tube and electric stirring device) and 4g thiopyridine trioxide compound (pentosan-pyridine trioxide ratio 1:5) was added, heated to 70 °C, stirring to dissolve. Then 0.8g pentosan was added to the threemouth flask, fully stirred and dissolved, and reacted at 70 °C for 6h. The reaction finishes and cools to room temperature. The pentosan sulfate was prepared by 3mol/L NaOH solution to PH=7, followed by precipitation with anhydrous ethanol, centrifugation, dialysis and lyophilization [22].
This method is easy to operate, generates less by-products, and can yield purer end products. However, the reagents are expensive, which is not conducive to industrial production.
Mechanical force chemistry method
The synthesis of pentosan sulfate by mechanical force chemistry method from sulfonic acid is a clean process invented by Prof. Zhang Long’s group in Jilin Provincial Engineering Laboratory for the Complex Utilization of Petro-resources and Biomass of Changchun University of Technology and has applied for a national invention patent. The group systematically investigated the clean process of extracting pentosan from wheat bran by hydrothermal method with high extraction rate of 100%. The process is characterized by simple operation, high product yield, high product quality, no use of toxic and harmful additives, and environmental friendliness, etc. It is an important reference for the green preparation of biomassbased sulfate and other derivatives. And then the mechanical chemical synthesis of pentosan is developed as follows:
4.0g of pentosan and 8.0g of sulfamic acid were placed in a ball miller in the ratio of 1:2 and reacted at room temperature for 12h at a ball milling speed of 600rmp. The product was dissolved in 10% NaOH solution, centrifuged to separate out the insoluble material, and the supernatant was taken into a three-neck flask, and the pH was adjusted to neutral by adding 30% hydrochloric acid solution dropwise with stirring for 30 min. Then add 3 times the volume of anhydrous ethanol to the above solution for alcoholic precipitation, leave it overnight, extract the precipitate, and freezedrying it to obtain the target product. The product was then refined by graded alcohol precipitation and finally freeze-dried at -40 °C and -0.095MPa to obtain pentosan sulfate and weighed. The yield of pentosan sulfate was 189.9% and the degree of sulfate substitution (DS) of the product was 1.714 [23].
Compared with other sulfonation processes, it has the advantages of milder process parameters, simple procedures and higher product quality with not production of “three wastes”, which is clean and meets the requirements of environmental protection [24], while the industrial production method still needs to be explored.
Analysis of the Biological Activities of Pentosan Sulfate
Pentosan itself has antitumor and antioxidant effects, but its application in biological activity is limited in many ways due to its large molecular weight. When pentosan is esterified, the sulfate group is introduced, which causes polysaccharide degradation and the biological activity will be more significant. Currently pentosan sulfate exhibits a variety of biological activities such as antitumor, antiviral, anticoagulant, antioxidant and anti-inflammatory [25].
Anticoagulant activity
It has been shown that the polysaccharide sulfate generated by the sulfated modification of polysaccharides have anticoagulant activity. Therefore, sulfated polysaccharides without health risks have become a new research hotspot. Both natural sulfated polysaccharides and chemically synthesized sulfated polysaccharides have good anticoagulant activity.
The carboxyl and sulfate groups on the pentosan sulfate molecule are important functional groups that bind antithrombin III and maintain the conformation and activity of antithrombin III. Antithrombin III can complex strongly with many coagulation factors, inhibit the activity of coagulation factors, and thus show good anticoagulant properties [26]. Pentosan sulfate itself does not possess anticoagulant activity, but mainly binds to the amino lysine residue on antithrombin III through the negatively charged sulfate group, changing the conformation of antithrombin III and accelerating its inactivation of coagulation factors [27].
It was found that glycosaminoglycans inhibit coagulation in two ways: one is that serine protease inhibitors (antithrombin III, heparin cofactor II) form 1:1 complexes with glycosaminoglycans such as heparin, acetyl heparin sulfate, and dermatan sulfate, which inactivate the coagulation factors or protein hydrolases (thrombin, etc.); the other is that protein C induces chondroitin sulfate glycosaminoglycans in thrombomodulin to cleave different attachment sites of coagulation factor Va and coagulation factor VIIIa to inactivate coagulation factors. Overall, glycosaminoglycans prevent coagulation by inhibiting coagulation factor activation as a pathway [28,29].
Anticoagulant biomaterials are an important part of biomaterials and are widely used in medical materials that come in contact with human blood and tissues, such as hemodialysis systems, extracorporeal circulation systems, artificial heart valves, pacemakers, artificial blood vessels, vascular stents, surgical procedures and catheters [30]. It has been shown that the modification of plant polysaccharides can obtain polysaccharide sulfate esters, which can be applied to the anticoagulant coating of pipeline materials and play a good anticoagulant effect. Plant polysaccharides were modified by sulfation and the modified polysaccharide sulfate coatings were immobilized on the surface of the in vitro circulation pipeline materials by covalent bonding method. Natural plant polysaccharides can have good anticoagulant properties after sulfation esterification, and they can effectively bond with the pipeline materials by covalent cross-linking to exert anticoagulant effects [31].
Antiviral activity
The results of recent studies have shown that pentosan sulfate inhibits the adsorption of viruses to cells and thus exerts antiviral activity [32]. Sulfated polysaccharides have antiviral activity and the sulfate group is a key component of the antiviral activity of some sulfated polysaccharides. After sulfated structural modification, the conformation of polysaccharides changes, and conformational changes are often the determinants of biological activity changes [33].
A large number of studies have shown that it is the sulfate group content that affects the antiviral activity of sulfate polysaccharides, and the sulfated structure is an important active part of polysaccharide antiviral, generally the higher the degree of sulfation, the stronger the antiviral activity. The polyanionic properties of sulfated polysaccharides are closely related to the distribution of their sulfate groups, and the degree of sulfate substitution and the position of substitution are important factors affecting their antiviral activity [34]. The antiviral activity of sulfated polysaccharide is related to the spatial structure of the sugar chain. After polysaccharide sulfation, the flexibility of the sugar chain is reduced. After the introduction of sulfate groups into the polysaccharide, the polymerization chains between the original polysaccharides were broken, and the conformation of the polysaccharide was extended. In addition, the repulsion between the sulfate groups leads to the distortion or transformation of the sugar ring conformation, so that the curled conformation of the polysaccharide is stretched and rigid, which may be the reason for the antiviral activity of the sulfated polysaccharide. In addition, after sulfation of polysaccharides, SO42- readily binds to specific structural domains of proteins, thus changing their conformation and affecting their function [35].
The antiviral mechanisms of polysulfate are broadly divided into four categories. First, it can either inhibit the infective ability of the virus by directly interacting with the surface of the virus through the negative charge it carries, or directly kill the virus so that it loses its infective ability. Second, polysulfate can inhibit viral transcription and replication both by directly interfering with viral replication-related enzymes and by acting on relevant targets within the host cell. Third, sulfated polysaccharides have strong polyanionic properties, which can bind to viruses or viral receptors on the cell surface, preventing viral adsorption or inhibiting a phase of the replication cycle after viral entry into the cell. Fourth, the immunomodulatory function of sulfated polysaccharides is mainly based on the regulation of macrophages, which induce the production of immune cytokines and can exert antiviral effects indirectly by activating innate immunity [36].
Viral diseases of livestock and poultry are a class of infectious diseases that seriously endanger livestock production, and some zoonotic diseases also pose a serious threat to human health. Therefore, the search and development of new antiviral agents with different mechanisms of action and low toxicity has become an issue of concern. The broad antiviral activity of poly-sulfates is expected to be another potential class of novel antiviral drugs after viral reverse transcriptase activity inhibitors and protease inhibitors [37]. The antiviral effect of pentosan polysulfate (PPS) has been evaluated and is currently approved or in clinical trials against dengue virus and encephalitis flavivirus, Japanese encephalitis virus, West Nile virus, and Murray Valley encephalitis virus [38].
Antitumor activity
Polysaccharides with antitumor activity generally contain sulfate groups, and the antitumor activity of natural polysaccharides is increased after structural modification by sulfate esterification. The polysaccharide glycosidic bonds are divided into α and β conformations, and generally the β conformation polysaccharide is more active. The (1,3) glycosidic bond on the glucose chain and the (1,6) glycosidic bond on the branched chain are required for antitumor activity [39].
Sulfate has inhibitory effects on a variety of tumors and cancer cells, and its mechanism of action is mainly divided into induction of apoptosis, inhibition of angiogenesis, cell cycle arrest and inhibition of cell migration. There are two main apoptotic pathways that have been studied, one is the exogenous pathway, i.e., the death receptor pathway, which activates intracellular apoptotic enzymes through extracellular signaling; the other is the endogenous pathway, i.e., the mitochondrial apoptotic pathway, which activates downstream cysteine proteases through the release of apoptotic factors from mitochondria, and the two pathways are interconnected, and ultimately both activate downstream effector molecules, caspases, by activating thereby causing activation of nucleases and degradation of important proteins [40].
At present, the research on the antitumor activity of sulfated polysaccharides is still at the surface stage, and the mechanism of antitumor action has not been elucidated, mostly in vitro experiments are conducted and in vivo experiments are less studied. Scholars need to make deeper research on sulfated polysaccharides, further determine the conformational relationship between their structure and function, clarify the mechanism of action, and conduct safety toxicology evaluation to ensure safety and stability, so that they are expected to become new drugs on the market [41].
Antioxidant activity
The antioxidant effects of polysaccharides of natural origin have been reported, and some findings suggest that sulfate polysaccharides of seaweed and sea cucumber origin can exert antioxidant effects by scavenging stable free radicals and superoxide anions and inhibiting polyphenol oxidase activity. We infer that the mechanism of the reaction between free radicals and polysaccharides may be that oxygen radicals first oxidatively degrade the glyoxylate portion of the sugar chain, and then sever the glycosidic bonds formed by mannose, galactose, and glucose on the sugar chain, thus degrading the sulfate polysaccharide, while the high-sulfur, high-amylose portion of the sulfate polysaccharide is not easily oxidized by oxygen radicals and therefore not easily degraded and de-sulfated [42].
Pentosan sulfate can bind to free radicals and superoxide anions and organize free radicals to attack other cells or tissues, thus exerting an antioxidant effect [43]. The antioxidant activity of polysaccharides is significantly increased after sulfation, probably because the hydroxyl groups of polysaccharide units are replaced by sulfate groups, the conformation of the sugar ring may be distorted or transformed and facilitate the formation of non-covalent bonds. The repulsion between these anionic groups elongates the sugar chain segments and some of the sulfate groups may form hydrogen bonds with the hydroxyl groups on the sugar ring, thus a helical structure may be formed locally in the sugar chain. The sugar chain is more extended and the orderliness is obviously increased. The viscosity is reduced and the binding ability to free radicals is increased [44].
Synthetic antioxidants have some serious toxic side effects on liver and kidney, and there are safety risks in their use, while natural antioxidants can avoid these side effects. Thus, for the prevention and treatment of diseases related to free radical imbalance, it is important to discover natural antioxidants that can effectively block oxygen radical reactions and have less toxic side effects [45].
Anti-inflammatory activity
Pentosan sulfate inhibited NF-kB, reduced the pro-inflammatory effects of TNFa, and decreased the production of MCP-1 stimulated by high glucose and AGEs. Meanwhile, pentosan sulfate reduced TNFa-induced cellular monolayer albumin permeability. Therefore, pentosan sulfate may inhibit inflammation by inhibiting TNFa, high glucose, advanced glycosylation end products (AGEs), and NF-kB in vitro [46].
Studies have shown that plant polysaccharides have therapeutic effects on inflammation by downregulating the phosphorylation levels of proteins involved in the inflammation-related NF-κB and MAPK signaling pathways, regulating the transcription of the inflammatory factors TNF-α and IL-1β, decreasing the secretion of the pro-inflammatory factor IL-6 and increasing the expression of the anti-inflammatory factor IL-10, and decreasing the expression of the proteins iNOS and COX-2 [47].
Oral pentosan polysulfate has been used for the treatment of painful bladder syndrome/interstitial cystitis for nearly 35 years, and oral pentosan polysulfate (PPS), a synthetic polysaccharide that is a close analogue of the bladder-protective glycosaminoglycan (GAG) layer, was approved by the FDA in the United States for the treatment of this disease [48].
Conclusion
Anticoagulant drugs need to have anticoagulant effect without side effects on human body, and pentosan sulfate is expected to be a new and ideal drug for promoting human health. In this paper, four preparation methods and antitumor, antiviral, anticoagulant, antioxidant and anti-inflammatory activities of pentosan sulfate are analyzed in detail, which provide useful references for the subsequent research of pentosan sulfate. However, the mechanism of action of pentosan sulfate bioactivities is not clear and needs to be further investigated.
At present, there is no systematic study on the preparation of pentosan sulfate in China and the relationship between its biological activity and conformation is not clear and most studies on its biological activity are focused on in vitro pharmacological activity studies. Therefore, further systematic and complete studies on pentosan sulfate should be focused on the development of green and environmentally friendly pentosan sulfate synthesis technology, the study of the quantitative relationship between the structures, substitution degrees and molecular weight of pentosan sulfates on their biological activity and the study and elaboration of in vivo activity are of great significance for the industrial preparation and commercial application of pentosan sulfates.
References
- Cui D, Liu M, Rui L, Bi Y (2010) Synthesis and optimization of the reaction conditions of starch sulfates in aqueous solution. Starch 59(2): 91-98.
- Zheng X, Jiang Q, Li X, Li L (2006) Study on the physicochemical properties of wheat bran pentosan. Journal of Henan University of Technology (Natural Science Edition) 6: 15-19.
- Izydorczyk MS, Biliaderis CG, Bushuk W (1990) Oxidative gelation studies of water-soluble pentosans from wheat. Journal of Cereal Science 11(2): 153-169.
- Dong B, Zheng X, Wang F (2005) Research progress of pentosan in wheat at home and abroad. Henan Agricultural Science 34(005): 8-10.
- Maity GN, Maity P, Dasgupta A, Acharya K, Dalai S, et al. (2019) Structural and antioxidant studies of a new arabinoxylan from green stem andrographis paniculata (kalmegh). Carbohydrate Polymers 212: 297-303.
- Erum A, Bashir S, Saghir S (2015) Modified and unmodified arabinoxylans from plantago ovata husk: novel excipients with antimicrobial potential. Bangladesh Journal of Pharmacology 10(4): 765.
- Men X, Wang Y, Kang Y, Zhu Y, Zhu L (2005) A comparative study on the optimal process conditions and properties of fucoidan sulfate from leaves and stems of Wakame. Food Industry Science and Technology (07): 160-162.
- Jindal M, Rana V, Kumar V, Sapra B, Tiwary AK (2013) Synthesis, physico-chemical and biomedical applications of sulfated aegle marmelos gum: green chemistry approach. Arabian Journal of Chemistry 10(S2): S2151-S2159.
- Lin L, Yu Y, Zhang F, Xia K, Zhang X, et al. (2019) Bottom-up and top-down profiling of pentosan polysulfate. Analyst 144(14): 4781-4786.
- William H (2017) Marine polysaccharide sulfatases. Frontiers in Marine Science 4: 6.
- Wijesinghe W, Jeon YJ (2012) Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydrate Polymers 88(1): 13-20.
- Adila A, Li J, Fu C, Zhou F, Li J (2019) Advances in the study of biological activity of polysaccharide sulfate. Journal of Food and Biotechnology 38(01): 7-13.
- Li X, Wang S, Liu F, Tang X (2021) Research progress of wheat bran pentosan extraction, efficacy and application. Journal of Food Safety (24): 120-123+125.
- Saqib A, Mubarik A, Qasim C, Abid H (2018) Effects of water extractable and unextractable pentosans on dough and bread properties of hard wheat cultivars. LWT 97: 736-742.
- Xue Y, Li C, Guan H (2014) Research progress on the determination of polysaccharide sulfate. Chinese Journal of Pharmaceutical Sciences 49(04): 271-274.
- Alekseeva A, Raman R, Eisele G, Clark T, Bertini S, et al. (2020) In-depth structural characterization of pentosan polysulfate sodium complex drug using orthogonal analytical tools. Carbohydrate Polymers 234: 115913.
- Wang Z, Li L, Guo S, Wei D (2004) Application of bioactive polysaccharides in cosmetics. Proceedings of the 2004 China Cosmetic Symposium, pp. 307-310.
- Fernando IPS, Kim KN, Kim D, Jeon YJ (2018) Algal polysaccharides: potential bioactive substances for cosmeceutical applications. Critical Reviews in Biotechnology 39(1): 99-113.
- Xu H, Han L, Xu Z (2008) Anti-coagulant and anti-platelet aggregation activity of corn cob xylan sulfate. Cellulose Science and Technology (3): 28-34.
- Wang P (2016) Study of process conditions for the preparation of pentosan sulfate from corn stover source. Changchun University of Technology, China.
- Shi F (2010) Progress of sulfate esterification reaction. Journal of Southeast University (Medical Edition) 29(6): 707-710.
- Mi H, Huang C, Sun C, Liu Q, Meng P, et al. (2014) Preparation process of pentosan sulfate from corn stover. Journal of Changchun University of Technology (Natural Science Edition) 35(6):716-719.
- Qin T (2019) Clean process for the extraction of pentosan and its derivatives preparation. Changchun University of Technology, China.
- Zhang Z, Wang X, Han L, Wang X (2007) Research progress of sulfonation reaction process. Chemical Propellants and Polymer Materials (01): 38-42.
- Degenhardt M, Gosh P, Tzig HW (2000) Studies on the structural variations of pentosan polysulfate sodium (NaPPS) from different sources by capillary electrophoresis. Arch Pharm 334(1): 27-29.
- Wen Z, Wu S, Chen W (2005) Research progress of heparinized anticoagulant polymer materials for medical use. Plastics 34(2): 26-30.
- Cheng H (2011) Isolation and characterization of corn straw hemicellulose and its modification by sulfuric acid esterification. South China University of Technology, China.
- Baumann H, Richter A, Klemm D, Faust V (2000) Concepts for preparation of novel regioselective modified cellulose derivatives sulfated, aminated, carboxylated and acetylated for hemocompatible ultrathin coatings on biomaterials. Macromolecular Chemistry and Physics 201(15): 1950-1962.
- Barddal H, Faria F, Nogueira AV, Iacomini M, Cipriani TR (2020) Anticoagulant and antithrombotic effects of chemically sulfated guar gum. International Journal of Biological Macromolecules 145: 604-610.
- He S, Zhou J (2010) Anticoagulant biomaterials. Progress in Chemistry 22(04): 760-772.
- Yang X, Yu M, Gao W (2019) Application of polysaccharide sulfate anticoagulation coating to polymer medical lines. Journal of Tianjin Medical University 25(02): 110-114.
- Baba M, Nakajima M, Schols D, Pauwels R, Balzarini J, et al. (1988) Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-hiv agent in vitro. Antiviral Research 9(6): 335-343.
- Ruan S (2012) Antiviral and immune-enhancing activities of sulfated polysaccharides from Nigella sativa and their comparison with nine other sulfated polysaccharides. Nanjing Agricultural University, China.
- Gou Q (2012) Constitutive relationship of antiviral activity of polysaccharide sulfate. Advances in Animal Medicine 33(12): 187-190.
- Chu H, Mao H, Liu R, Geng Y (2010) Progress of antiviral activity of sulfated polysaccharides and their conformational relationships. Science and Technology Information (36): 507-508.
- Wu L, Zhao X, Wang W (2016) Mechanism and constitutive relationship of antiviral action of marine sulfate polysaccharide. China Marine Drug 35(04): 87-92.
- Chen J, Hu T (2005) Advances in the antiviral effects of polysaccharide sulfate. Advances in Animal Medicine (04): 54-57.
- Lee E, Pavy M, Young N, Freeman C, Lobigs M (2006) Antiviral effect of the heparan sulfate mimetic, pi-88, against dengue and encephalitic flaviviruses. Antiviral Res 69(1): 31-38.
- Qiu L, Xin X, Geng M (2009) Progress in the study of polysaccharide conformational relationships. Modern Biomedical Progress 9(09): 1764-1768.
- Bai X, Hu B, Song S, Hao L, Ji A (2019) Antitumor mechanism of rockulose sulfate. Chemistry of Life 39(5):6.
- Zeng M, Zhou B, Guo X (2018) Advances in anti-tumor research of sulfated polysaccharides. Chinese Pharmacist 21(06): 1078-1082.
- Zhao X, Dong S, Sun L, Li F, Li B (2011) Oxygen radical scavenging activity and mechanism of kelp polysaccharides. Journal of Aquaculture 35(04): 531-538.
- Hou L, Chen X, Sun Y, Liu N (2017) Study on the effect of sulfated apple polysaccharides on free radical scavenging. Food Science and Technology 42(02): 188-192.
- Yang W, Yuan C, Han H (2021) Preliminary study on the in vitro antioxidant activity of marine sulphate polysaccharides. Natural Products Research and Development 33(07): 1081-1085.
- Li F, Wang W, Xu W, Zhang H, Zhang X (2014) Optimization of sulfate esterification process and antioxidant activity of corn husk polysaccharides. Chinese Journal of Cereals and Oils 29(11): 104-107+112.
- Wu J, Guan TJ, Zheng S, Grosjean F, Liu W, et al. (2011) Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging c57b6 mice. Laboratory investigation; A Journal of Technical Methods and Pathology 91(10): 1459-71.
- Miao J, Qiu J, Li H, Yu B, Zhou D, et al. (2019) Research progress on the effect of natural polysaccharides on the regulation of intestinal flora. China Food and Nutrition 25(12): 52-58.
- Taneja R (2021) Current status of oral pentosan poly-sulphate in bladder pain syndrome/interstitial cystitis. International Urogynecology Journal 32: 1107-1115.
© 2023 Long Zhang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






