- Submissions

Full Text
Research in Medical & Engineering Sciences
Utricle with Big C-Follicular T cell lymphoma
Anubha Bajaj*
Department of Histopathology, AB Diagnostics, New Delhi, India
*Corresponding author: Anubha Bajaj, Department of Histopathology, AB Diagnostics, New Delhi, India
Submission: February 10, 2023Published: February 15, 2023

ISSN: 2576-8816Volume10 Issue2
Opinion
Follicular T cell lymphoma is an exceptional subtype of nodal lymphoma emerging from T follicular helper (Tfh) cells. The lymphoma demonstrates a nodal, follicular or perifollicular pattern of neoplastic configuration wherein neoplastic cells exhibit an intense, perpetual expression of T follicular helper immune markers. Follicular T cell lymphoma may be associated with Epstein Barr viral infection. Follicular T cell lymphoma manifests clinical and diagnostic features simulating angioimmunoblastic T cell lymphoma (AITL). Appropriate discernment of extra-nodal follicular T cell lymphoma can be challenging, in the absence of categorical follicular T cell lymphoma implicating lymph nodes.
Additionally designated as follicular peripheral T cell lymphoma (F-PTCL), follicular T cell lymphoma (FTCL), peripheral T cell lymphoma with nodular, follicular or perifollicular pattern or peripheral T cell lymphoma with follicular involvement, follicular T cell lymphoma manifests ~2% of T cell lymphomas. Middle aged and elderly individuals are commonly incriminated. A mild male predominance is observed. Upon flow cytometry, an aberrant T cell population may be identified. T cell population commonly depicts an immuno-phenotype of CD3- / dim CD4+ T, especially discerned within follicular subtype of peripheral T cell lymphoma [1,2].
Characteristic morphological features are denominated as a follicular or perifollicular pattern of tumour evolution. Nevertheless, histologic features commonly discerned within angioimmunoblastic T cell lymphoma as proliferation of venules layered with plump endothelium, polymorphous inflammatory infiltrate confined to inter-follicular zones or expansive networks of follicular dendritic cells are absent [1,2].
Generally, dual or triple immune markers indicative of T follicular helper cells are consistently expressed. An estimated one fifth instances of follicular T cell lymphoma manifest chromosomal translocation t(5;9)(q33;q22) with ITK-SYK genetic rearrangement.
Follicular T cell lymphoma commonly represents as disseminated or advanced stage disease with incrimination of regional or distant lymph nodes. Extra-nodal sites as cutaneous surfaces, bone marrow, hepatic parenchyma or spleen are infrequently implicated [1,2].
Follicular T cell lymphoma simulates clinical, genetic and morphological features along with molecular signatures of angioimmunoblastic T cell lymphoma, thereby indicating a common pathogenesis. Akin to angioimmunoblastic T cell lymphoma, follicular T cell lymphoma enunciates molecular anomalies within T follicular helper cells of origin as genomic aberrations within RHOA, TET2, IDH2 and DNMT3A genes.
Cytogenetic analysis depicts chromosomal translocation t (5;9) (q33; q22) with consequent ITK-SYK genetic fusion emerging in an estimated 20% instances [1,2]. Majority (~100%) of follicular T cell lymphoma manifest with monoclonal rearrangement of T cell receptor genes. However, clonal immunoglobulin heavy chain (IgH) genetic rearrangements are variably encountered. Targeted next generation sequencing analysis delineates frequent chromosomal mutations, especially within TET2, RHOA, DNMT3A and IDH2 genes. Also, RHOA Gly17Val chromosomal mutation may appear with angioimmunoblastic T cell lymphoma and peripheral T cell lymphoma arising from T follicular helper cells (PTCL-TFH) [1,2]. Follicular T cell lymphoma represents with generalized lymph node enlargement, splenomegaly, B symptoms as pyrexia, night sweats, >10% loss of body weight or a cutaneous eruption. Occasionally, localized presentation of an indolent or protracted disease may be observed [1,2].
Cytological examination depicts a variable cellular countenance
and appropriate detection of follicular T cell lymphoma singularly
upon cytological features may be challenging. Typically, the cellular
neoplastic infiltrate represents with monotonous dissemination
of neoplastic lymphoid cells incorporated with abundant, pale
staining cytoplasm, vesicular nuclei and prominent nucleoli.
Cellular atypia is minimal. Hodgkin’s Reed Sternberg (RS)-like cells
or epithelioid histiocytes appear in a majority of instances [1,2].
Microscopic examination of incriminated lymph node delineates
distinct architectural configurations denominated as
A. Progressive transformation of germinal centre (PTGC)-
like pattern exemplifies well defined, cellular aggregates of
neoplastic T cells intermingled with numerous B lymphocytes
incorporated with immunoglobulin D (IgD), simulating foci of
progressive transformation of germinal centre upon routine
haematoxylin and eosin (H&E) stain.
B. Follicular lymphoma-like pattern is frequently discerned
and manifests neoplastic cells which articulate intra-follicular
cellular aggregates or neoplastic nodules confined within
lymphoid follicles. Mantle zone may frequently be attenuated
or absent.
Besides, follicular lymphoma-like and progressive transformation of germinal centre -like patterns are frequently commingled in a singular neoplasm. Neoplastic cells emerge as monotonous cells of intermediate magnitude pervaded with moderate to abundant, clear or pale eosinophilic cytoplasm, vesicular nuclei or coarse, granular nuclear chromatin and prominent nucleoli. Cellular atypia is minimal [2,3]. Inter-follicular zones appear devoid of characteristic polymorphic inflammatory infiltrate and vascular proliferation as observed within lymph nodes incriminated with angioimmunoblastic T cell lymphoma. However, enlarged B cells, Hodgkin’s Reed Sternberg (RS)-like cells or epithelioid histiocytes are commonly confined to inter-follicular areas [2,3].
Classically, cutaneous lesions manifest with an intense lymphocytic inflammatory infiltrate configuring nodules and comprehensively invading diverse cutaneous surfaces. Aforesaid neoplastic T lymphocytes appear admixed with histiocytes. The inflammatory infiltrate variably expands into subjacent subcutaneous adipose tissue [2,3]. Follicular T cell lymphoma manifests an interstitial or para-trabecular pattern of tumour dissemination within the bone marrow. Incriminated extra-nodal, mucosal sites appear concurrent with lympho-epithelial lesions, as encountered within extra-nodal marginal zone B cell lymphoma arising from mucosa associated lymphoid tissue (MALT lymphoma) [2,3].
Lugano classification of staging non Hodgkin’s lymphoma is
denominated as
a) stage I is described as
~lymphoma confined to singular lymph node region (I)
~lymphoma incriminates singular extra-lymphatic organ or
extra-nodal site whereas lymph node involvement is absent (IE)
b) stage II is described as
~lymphoma incriminating ≥2 lymph node regions on one
side of diaphragm (II) ~lymphoma incriminates singular
organ and regional lymph nodes along with or absence of
neoplasm confined to diverse lymph node regions on one side
of diaphragm (IIE)
c) stage III where lymphoma incriminates lymph node
regions on opposite sides of diaphragm (III)
d) stage IV where lymphoma disseminates to diverse extralymphatic
organs as bone marrow, hepatic or pulmonary
parenchyma along with or devoid of incrimination of various
lymph node groups (IV) [2,3].
Follicular T cell lymphoma is immune reactive to pan T cell antigens such as CD2, CD3, CD5 along with frequently decimated CD7 expression. Neoplastic lymphocytes depict a helper T cell phenotype as CD4+/CD8-. Exceptionally, CD4-/CD8- immunophenotype may occur. T follicular helper cell immune markers as PD-1, CXCL13, BCL6, CD10 and ICOS are denominated wherein PD-1 or ICOS appear comprehensively immune reactive. The majority of neoplasms are immune reactive to CD10, BCL6 or CXCL13. Immune reactivity to CD21, CD23 and CD35 appears within remnant follicles. Hodgkin’s Reed Sternberg-like enlarged cells appear immune reactive to CD15, CD30 or PAX5 and frequently demonstrate Epstein Barr viral infection [3,4]. The Ki67 proliferative index is variable and ranges from 5% to 70% with median and mean Ki67 index at ~45%. Follicular T cell lymphoma is immune non-reactive to CD56 and cytotoxic antigens as T cell intracellular antigen 1(TIA1) and granzyme B [3,4].
Follicular T cell lymphoma requires segregation from neoplasms such as angioimmunoblastic T cell lymphoma, peripheral T cell lymphoma, classic Hodgkin’s lymphoma, follicular lymphoma, methotrexate associated lymphoproliferative disorder with features of follicular T cell lymphoma and reactive lymph node alterations or paracortical hyperplasia [3,4]. Follicular T cell lymphoma may be appropriately discerned with an amalgamation of morphological and immuno-phenotypic assessment of surgical tissue samples, preferably obtained from incriminated lymph nodes. The lymphoma demonstrates a mature T follicular helper phenotype wherein diagnostic criterion of angioimmunoblastic T cell lymphoma (AITL) as defined by World Health Organization appear lacking (Figures 1&2) [5,6].
Figure 1:Follicular T cell lymphoma depicting nodules of neoplastic lymphocytes with moderate, pale cytoplasm, vesicular nuclei and prominent nucleoli admixed with Reed Sternberg-like cells and epithelioid histiocytes. Neoplastic T lymphocytes are immune reactive to PD-1 and CD20 [5].
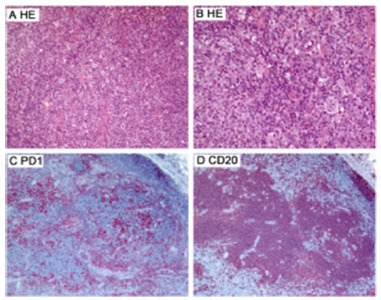
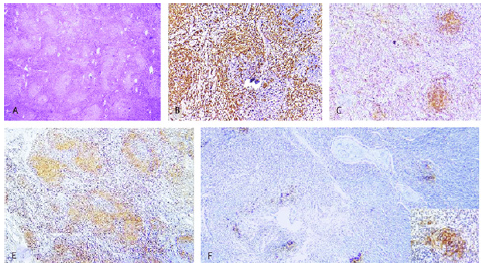
Figure 2:Follicular T cell lymphoma depicting effaced lymph node architecture with follicular configuration. Neoplastic lymphocytes of intermediate size display abundant, pale cytoplasm, vesicular nuclei and prominent nucleoli with admixed inflammatory cell infiltrate. Neoplastic T lymphocytes are immune reactive to diverse T cell markers [6].
Appropriate disease staging may be obtained with bone marrow biopsy. Follicular T cell lymphoma may occasionally depict hypergammaglobulinaemia with reactive Coombs test, in contrast to characteristic association as encountered with angioimmunoblastic T cell lymphoma (Table 1).
Table 1World Health Organization (2017) classification of T follicular helper cell lymphomas [2,3].

concurrence with therapeutic outcomes, segregation between follicular T cell lymphoma and peripheral T cell lymphoma not otherwise specified (PTCL-NOS) may appear superfluous. Clinical management of dual conditions appears identical. Contemporary therapeutic strategies and targeted chemotherapies as hypomethylating agents can be advantageously adopted. Therapeutic induction may be obtained with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or anthracycline based regimens as cyclophosphamide, doxorubicin, etoposide, vincristine and prednisone (CHOEP) or etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (EPOCH). Consolidation therapy as an autologous stem cell transplantation may be employed in responsive individuals. Preferential second line agents as pralatrexate, romidepsin, belinostat and brentuximab vedotin may be adopted for treating relapsed or refractory disease [3,4]. Typically, follicular T cell lymphoma manifests with an aggressive clinical course. An estimated 50% mortality is encountered within the initial 24 months of disease discernment [3,4].
References
- Satou A, Takahara T, Tsuzuki T (2022) Pathological and molecular features of nodal peripheral t-cell lymphomas. Diagnostics (Basel) 12(8): 2001.
- Krug A, Tari G, Saidane A, Gaulard P, Ricci JE, et al. (2022) Novel T follicular helper-like t-cell lymphoma therapies: From preclinical evaluation to clinical reality. Cancers (Basel) 14(10): 2392.
- Wang L, Rocas D, Dalle S, Sako N, Pelletier L, et al. (2022) Primary cutaneous peripheral T-cell lymphomas with a T-follicular helper phenotype: an integrative clinical, pathological and molecular case series study. Br J Dermatol 187(6): 970-980.
- Yoon SE, Cho J, Kim YJ, Ko YH, Park WY, et al. (2021) Comprehensive analysis of clinical, pathological, and genomic characteristics of follicular helper T-cell derived lymphomas. Exp Hematol Oncol 10(1): 33.
- Figure 1 Courtesy: Nature.com
- Figure 2 Courtesy: Research gate
© 2023 Anubha Bajaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






