- Submissions

Full Text
Research in Medical & Engineering Sciences
Mecobalamin as A Neuroprotective Effector Against Oxaliplatin-Based Chemotherapy in Colon and Rectal Cancer Patients
Fei Li*, Hongyan Li and Wanting Lu
General Surgery Department, Capital Medical University, China
*Corresponding author: Fei Li, General Surgery Department, Xuan Wu Hospital, Capital Medical University, China
Submission: November 11, 2020Published: March 16, 2021

ISSN: 2576-8816Volume9 Issue2
Abstract
Palliative chemotherapy prolongs survival and improves quality of life. However, a variety of chemotherapeutics including oxaliplatin can cause severe side effects during treatments, leading to painful symptoms that might result in the interruption of cancer treatment. Although adding oxaliplatin to fluorouracil and leucovorin in adjuvant chemotherapy for colon and rectal cancer may improve disease-free survival, it also increases grade 3-4 sensory neuropathy. Our study aimed to determine whether oral Mecobalamin is neuroprotective against oxaliplatin-induced neuropathy. Forty-six stage III colon and rectal cancer patients receiving adjuvant biweekly oxaliplatin were randomized to oral Mecobalamin (1,500mg; case group) or placebo (control group). Clinical neurological and electrophysiological evaluations were performed at baseline and after 4, 8, and 12 treatment cycles. Treatment-related toxicity was evaluated based on National Cancer Institute (NCI) criteria. After four cycles of chemotherapy, 9 of 23 patients in the control group and 8 of 23 patients in case group experienced grade 1 sensory neuropathy. After eight cycles, 13 patients experienced sensory neuropathy (grade 2-4 toxicity) in the control group; however, no patients in the case group experienced sensory neuropathy (P<0.05). After 12 cycles, grade 2–4 sensory neuropathy was observed in 20 patients in the control group, but only in 4 patients in the case group (P<0.05). We did not observe any significant electrophysiological changes in the case group after 4, 8, or 12 cycles of chemotherapy. Thus, we demonstrated that oral Mecobalamin reduces the incidence of neuropathy in colon and rectal cancer patients receiving oxaliplatin-based adjuvant chemotherapy
Introduction
Colorectal cancer accounts for 10 to 15% of all cancers and is the second leading cause of cancer deaths [1]. Approximately half of all patients of colorectal cancer develop metastatic disease over time. Palliative chemotherapy in these patients prolongs survival and improves quality of life. In the last decade, development of novel therapies that target critical biologic pathways has greatly expanded treatment options for patients with Metastatic Colorectal Cancer (mCRC) and has shown substantial improvement in Progression-Free Survival (PFS). Oxaliplatin is a third-generation platinum drug and is widely used as a first-line therapy for the treatment of Colorectal Cancer (CRC). However, a variety of chemotherapeutics including oxaliplatin can cause severe side effects during treatments, leading to painful symptoms that might result in the interruption of cancer treatment [2-4]. Chemotherapy-Induced Peripheral Neuropathy (CIPN) is one of the most severe side effects of anticancer agents, such as platinum- and taxanes-derived drugs (oxaliplatin, cisplatin, carboplatin, and paclitaxel). CIPN is a debilitating and dose-dependent side effect caused by anticancer agents that interferes with cancer therapy regimens and affects long-term quality of life [5,6].
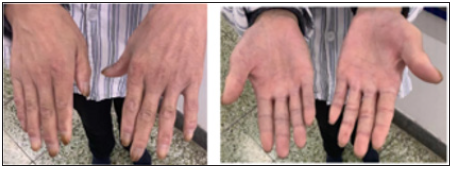
Taxanes, vinca alkaloids, platinum derivatives, bortezomib, and thalidomide are the most frequent agents causing CIPN. CIPN has symptoms such as pain, allodynia, loss of sensation, paresthesia, numbness, tingling, and gait disturbance [7]. These drugs predominantly impair afferent sensory fibers with a symmetric, distal, and length-dependent “glove and stocking” distribution (Figure 1). Thus, it is important to develop analgesic drugs to avoid the painful side effects of chemotherapy in colorectal cancer patients.
Figure 1: Chemotherapy-induced neuropathy causes a symmetric, distal, and length-dependent “glove and stocking” distribution in the periphery.
Oxaliplatin, a third-generation platinum compound that differs from previous platinum compounds, combined with fluorouracil (5FU), has been well established as first-line or salvage therapy in advanced colorectal cancer patients [8]. In a recent study, oxaliplatin-when combined with 5-FU and leucovorin (LV) to treat colorectal cancer patients in the adjuvant setting-improved disease-free survival but at the same time increased the incidence of grade 3 to 4 sensory neuropathy. Thus, only 62.5–74.7% of patients were able to complete the planned 12 cycles of oxaliplatinbased chemotherapy [9]. In fact, the most common dose-limiting toxicity resulting from oxaliplatin therapy is neurotoxicity. For most patients, oxaliplatin can induce unique acute peripheral sensory and motor toxicity with initial symptoms of paresthesia or coldrelated dysesthesia during or within hours following oxaliplatin infusion. This may induce significant disability in patient’s activity and affect compliance with treatment recommendations for colorectal cancer patients [10]. A likely mechanism underlying oxaliplatin-induced neurotoxicity is that an oxaliplatin metabolite such as oxalate may alter the properties of voltage-gated sodium channels or slowdown the clearance of platinum compounds from the peripheral nervous system [11,12]. Oral Mecobalamin is a relatively cheap, convenient, and safe oral drug, which has been used as a vitamin B12 supplement. To determine whether oral Mecobalamin prevents oxaliplatin-induced neuropathy, we studied its efficacy in reducing neuropathy in colorectal and rectal cancer patients receiving postoperative adjuvant oxaliplatin combined with 5-FU/LV chemotherapy.
Patients and Methods
Patients
From January 2018 to January 2020, a total of 46 stage III colorectal and rectal cancer patients receiving postoperative adjuvant oxaliplatin combined with a 5-FU/ LV regimen at Xuanwu Hospital were eligible for this study. All patients signed the consent form and were eligible for the study if they fulfilled the following criteria before treatment: Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, normal bone marrow function (white blood count ≥4,000/mm3, platelet count ≥100,000/mm3), liver function (serum total bilirubin<1.5mg/dl), renal function (creatinine<1.5mg/dl), and heart function (stable cardiac rhythm, no active angina, no clinical evidence of congestive heart failure). Patients were excluded during screening if they had clinical neuropathy, diabetes mellitus, alcoholic disease, brain involvement, or if they were on vitamin B1, B6, or another vitamin supplemental therapy. This study was approved by the hospital ethics Committee.
Medicine
The chemotherapy regimen consisted of biweekly oxaliplatin (85mg/m2), a weekly bolus of 5-FU (425mg/m2), and a lowdose LV (20mg/m2). All patients were randomized (1:1) to an oral Mecobalamin (1,200 mg) or placebo (control) group. Oral Mecobalamin (1,500mg) was given one and a half hours before each oxaliplatin administration in the case group. Oral placebo agents were given in the control group. Patients were not allowed to take any other sensory neuromodulatory agents such as calciummagnesium infusions or antiepileptic like agents.
Sensory neuropathy assessment and doses reduction
Toxicity due to clinical sensory neuropathy was assessed every two weeks using the common toxicity criteria (CTC) of the National Cancer Institute (NCI). If patients had NCI grade 2 sensory neuropathy for more than 14 days, the oxaliplatin dose was reduced to 75% of the original dose. If patients had grade 3 to 4 sensory neuropathy, oxaliplatin was discontinued until recovery. Complete neurological and electrophysiological examinations were performed by a neurologist. All patients were examined before the study and after 4, 8, and 12 cycles of chemotherapy, within two weeks of the end of treatment.
Data analysis
The primary endpoint of the study was to investigate the neuroprotective effect of oral Mecobalamin in oxaliplatin-induced neurotoxicity. Fisher’s exact test was used to assess differences in clinical neurotoxicity between the two groups. A modified calculation score that was derived from the sum of the degrees of the worst neurological toxicity in each patient according to the NCI scale and divided by the number of assessable patients for each dose level (400, 800, and 1,200mg per day) was used. The Mann-Whitney test was used to compare the electrophysiological results after 4, 8, and 12 cycles of chemotherapy. All analyses were performed using SPSS 13.0 software. Statistical significance was defined as P<0.05.
Results
Patient
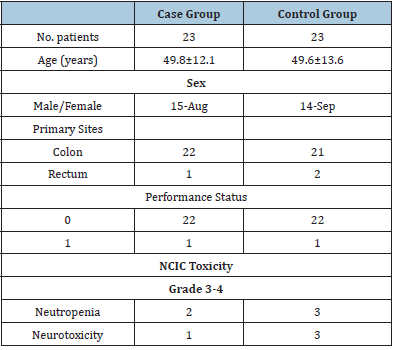
The patients’ characteristics are listed in Table 1. All patients were randomized to oral Mecobalamin (1,200mg; case group, N=23) or placebo (control group, N=23). The median ages in the Mecobalmin and placebo groups were 49.8±12.1 years and 49.6±13.6 years, respectively. The sex ratio (male: female) was 15:8 and 14:9 in the Mecobalmin and placebo groups, respectively. The overall percentage of grade 3 to 4 neutropenia and neurotoxicity was 10.9 and 8.7% in the Mecobalmin and placebo groups, respectively. No patients were excluded from the study during the study period.
Table 1:Patient characteristics and neurotoxicity results based on NCIC toxicity grading.
Clinical data
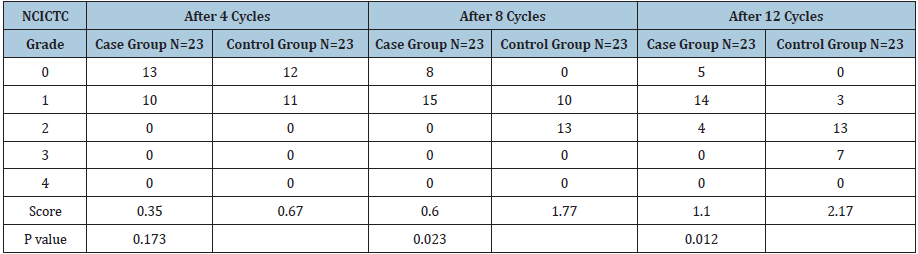
After four cycles of chemotherapy, 10 patients (43.5%) in the case group had clinical grade 1 neuropathy compared with 11 patients (47.8%) in the control group (P=0.173). After eight cycles of chemotherapy, 15 patients in the case group (65.2%) had grade 1 neurotoxicity and none had grade 2 to 4 neurotoxicity; 10 patients (43.4%) in the control group had grade 1 neurotoxicity and 13 (56.5%) patients had grade 2 to 4 neurotoxicity (P=0.023). After 12 cycles of chemotherapy, 14 patients in the case group (60.9%) had grade 1 and four patients (17.4%) had grade 2 to 4 neurotoxicity; however, in the control group, 3 patients (13%) had grade 1 and 20 patients (87%) had grade 2 to 4 neurotoxicity (P=0.012) (Table 2).
Table 2:Clinical effects of Mecobalamin on oxaliplatin-induced neurotoxicity.
Electrophysiological data
Electrophysiological evaluations showed no significant change in mean latency, sensory amplitude potentials, and conduction velocity of sural nerves in patients receiving Mecobalamin following 4, 8, and 12 cycles of chemotherapy (Table 3). Likewise, there were no significant motor electrophysiological changes in distal latency, compound muscle action potentials (CMAP) amplitude, nerve conduction velocity, and F wave latency (Table 4).
Table 3:Sensory electrophysiological results in the Mecobalamin arm.
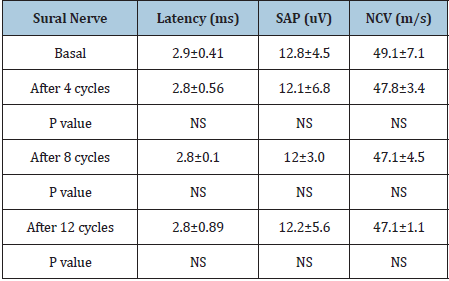
SAP: Sensory Amplitude Potential; NCV: Nerve Conduction Velocity; NS: Not significant
Table 4:Motor electrophysiological results in the Mecobalamin arm.
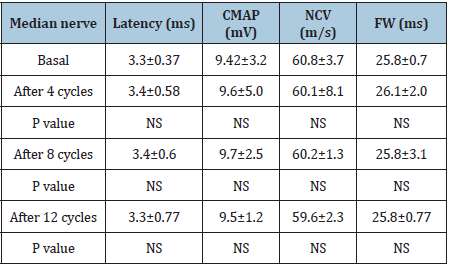
CMAP: Compound Muscle Action Potential; NCV: Nerve Conduction Velocity; FW: F wave latency; NS: Not significant
Discussion
Our study demonstrated that oral Mecobalamin can prevent
oxaliplatin-induced neuropathy. The incidence of clinical
neurotoxicity in patients receiving Mecobalamin was significantly
lower than in the placebo group despite 4, 8, and 12 cycles of
oxaliplatin-based therapy. Patients’ neurological symptoms did
not rebound and did not recur even after ceasing oral intake of
Mecobalamin. Oxaliplatin-induced neurotoxicity is characterized
by a rapid-onset acute sensory neuropathy and a late-onset
cumulative sensory neuropathy that usually occurs after several
cycles of therapy and is the most frequent dose limiting toxicity.
Mecobalamin is currently considered one of the most promising
cancer chemo-preventive agents. Oral Mecobalamin is a convenient,
safe oral drug. Mecobalamin can prevent oxaliplatin-induced
neuropathy without affecting the clinical activity of oxaliplatin [13].
The pharmacokinetics in patients receiving an oral 1,500mg dose
of Mecobalamin can result in good bioavailability and good plasma
concentration status [14,15].
Occasionally, clinical sensory neuropathy does not correlate
with findings of nerve conduction studies. It has been reported that
although the symptoms of oxaliplatin induced neuropathy reduced
after stopping administration of oxaliplatin, the abnormalities
of sensory nerve conduction were still persistent [16]. More
severe clinical grade 2 to 4 neuropathy and more sensory axonal
neuropathy, leading to abnormalities in sensory nerve conduction,
were observed in another study [17]. In our study, we did not
find any significant change in electrophysiological testing results
including sensory and motor nerve studies after 4, 8, and 12 cycles
of chemotherapy in the case group (Table 3 & 4). We ascribe this
alleviation of neuropathy to the use of Mecobalamin.
Our results indicate that oral Mecobalamin may protect against
oxaliplatin-induced sensory neurotoxicity and axonal neuropathy.
Mecobalamin may be useful in the prevention of oxaliplatininduced
neuropathy in the future. Due to the limited number of
patients in our study, a larger study should be conducted to confirm
our findings and to check the bioavailability of oral Mecobalamin
for prevention of oxaliplatin-induced neurotoxicity.
Acknowledgment
We thank LetPub (www.letpub.com) for its linguistic assistance and scientific consultation during the preparation of this manuscript.
Financial Support
Supported by Beijing Municipal Administration of Hospitals’ Youth Programme (QMS20180805); Support Project of Highlevel Teachers in Beijing Municipal Universities in the Period of 13th Five–year Plan (CIT&TCD201904093); Beijing Excellent Talents Training Funding Project (2018000026833ZK78); Xuanwu Hospital Huizhi Talent Project (XW2019091680124).
References
- Chamberlain MC (2010) Neurotoxicity of cancer treatment. Curr Oncol Rep 12(1): 60-67.
- Hou S, Huh B, Kim HK, Kim KH, Abdi S (2018) Treatment of chemotherapy-induced peripheral neuropathy: Systematic review and recommendations. Pain Physician 21(6): 571-592.
- Han Y, Smith MT (2013) Pathobiology of cancer Chemotherapy-Induced Peripheral Neuropathy (CIPN). Front Pharmacol 4: 156-163.
- Ben NM, Slomianka L, Vyssotski AL, Lipp HP (2010) Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 31(1): 151-161.
- Areti A, Yerra VG, Naidu V, Kumar A (2014) Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol 2: 289-295.
- Barton DL, Wos EJ, Qin R, Mattar BI, Green NB, et al. (2011) A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer 19(6): 833-841.
- Brown TJ, Sedhom R, Gupta A (2019) Chemotherapy-induced peripheral neuropathy. JAMA Oncol 5(5): 750.
- Shin HR, Masuyer E, Ferlay J, Curado MP, Asian contributors to CI5 IX4 (2010) Cancer in Asia-Incidence rates based on data in cancer incidence in five continents IX (1998-2002). Asian Pac J Cancer Prev 11(Suppl 2): 11-16.
- Heinonen H, Aro AR, Aalto AM, Uutela A (2004) Is the evaluation of the global quality of life determined by emotional status? Qual Life Res 13(8): 1347-1356.
- Grisold W, Cavaletti G, Windebank AJ (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro Oncol 14(Suppl 4): 45-54.
- A de Gramont, A Figer, M Seymour, M Homerin, A Hmissi, et, al. (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16): 2938-2947.
- André T, Boni C, Boudiaf LM, Navarro M, Tabernero J, et, al. (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23): 2343-2351.
- Raymond E, Chaney SG, Taamma A, Cvitkovic E (1998) Oxaliplatin: A review of preclinical and clinical studies. Ann Oncol 9(10): 1053-1071.
- Grolleau F, Gamelin L, Celle MB, Lapied B, Pelhate M, et al. (2001) A possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltagegated sodium channels. J Neurophysiol 85(5): 2293-2297.
- Cavaletti G, Tredici G, Petruccioli MG, Dondè E, Tredici P, et al. (2001) Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer 37(18): 2457-2463.
- Krishnan AV, Goldstein D, Friedlander M, Kiernan MC (2005) Oxaliplatin induced neurotoxicity and the development of neuropathy. Muscle Nerve 32(1): 51-60.
- Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK (2004) Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve 29(3): 387-392.
© 2021 Fei Li. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






