- Submissions

Full Text
Research in Medical & Engineering Sciences
Primary Angle Closure Glaucoma: A Review
Rodiah Rahmawaty Lubis1*, Nova Arianti2 and Novie Diana Sari3
1Department of Ophthalmology, Faculty of Medicine, Universitas Sumatera Utara, Indonesia
2Department of Ophthalmology, Pirngadi General Hospital, Indonesia
3Department of Ophthalmology, Haji Adam Malik General Hospital, Indonesia
*Corresponding author: Rodiah Rahmawaty Lubis, Department of Ophthalmology, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Submission: September 09, 2020 Published: September 24, 2020

ISSN: 2576-8816Volume9 Issue1
Abstract
Glaucoma is a leading cause of irreversible blindness worldwide. Primary angle closure glaucoma (PACG), is the second most common form of glaucoma and associated with the closure of the anterior chamber angle. Peripheral anterior synechia (PAS) frequently develop due to an attack of prolonged acute attack or a series subacute attack of angle closure glaucoma. These PAS are usually high and broad and very difficult to find out whether the PAS formed before or during the attack or possibly at both times. Principal management strategy for PACG is about anterior chamber angle by understanding the early stages of appositional angle closure before the PAS. In this review, we describe the current understanding regarding the pathophysiology and treatment of PACG.
Introduction
Globally at least 2,2 billion people have a vision impairment and glaucoma is the leading cause of global irreversible blindness. Vision impairment and blindness caused by glaucoma are irreversible but effective treatments and surgical interventions are available which can either delay or prevent progression of the disease [1]. Glaucoma is a group of diseases defined by an optic neuropathy, remodelling of the connective tissue elements of the optic nerve head, loss of neural tissue and development of visual field dysfunction. Classically, glaucoma classified into open-angle, angle-closure and childhood glaucoma. Angle-closure refers to an anatomical configuration in which there is mechanical blockage of trabecular meshwork by the peripheral iris. This anatomical configuration can obstruct iridocorneal drainage angle through apposition or formation of peripheral anterior synechiae (PAS). This anatomical configuration can differ open-angle and angle-closure, that is anatomical blockage of iridocorneal angle was not found in open-angle [2].
Figure 1: Three mechanisms of angle closure (a) at the level of pupil (b) at the level of iris and cilliary body (c) at the level of lens.
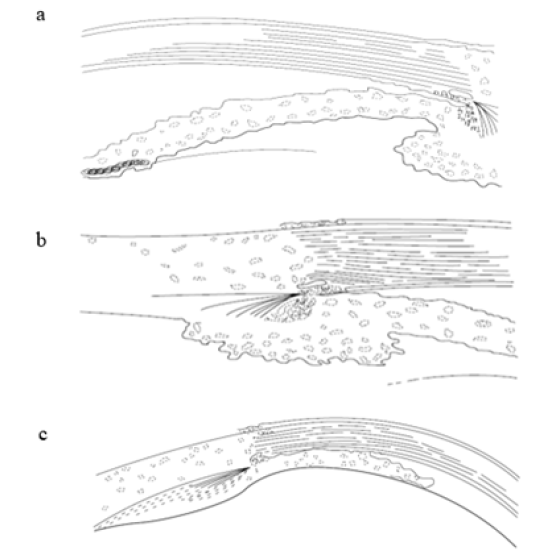
Figure 2: Signs of acute primary angle closure: conjunctival injection and mid-dilated pupil (left), iris sectoral atrophy (center) and glaucomflecken (right).
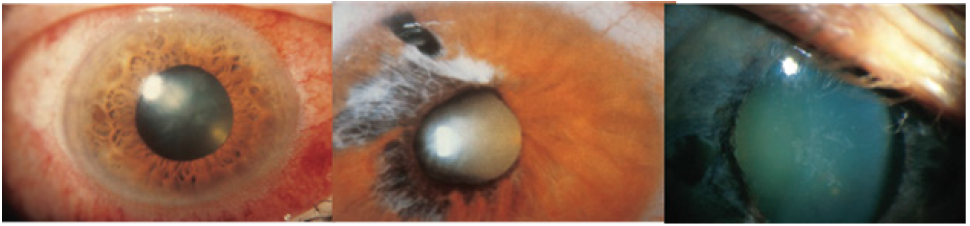
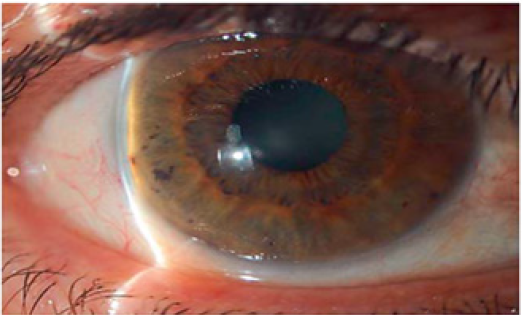
Figure 3: The van Herick Method [5].
Epidemiology
WHO reported that 76 million people suffered from glaucoma worldwide by 2020? 1The global prevalence of PACG and POAG were 0,50% and 3,05%, but there is variation of distribution among different ethnic group. Asian had the highest prevalence of PACG rather than other ethnics. In 2020, 23.36 million people suffered from PACG and it was estimated that it will increase to 32.04 million people in 2040 [3].
Risk factor
PACG more often found in Asian rather than other ethnics. The prevalence of PACG in Asian is 17.96 million people, contribute more than 76% of PACG worldwide in 2020. Ocular biometric predisposed eye to develop PACG. Shallow anterior chamber depth less than 2.5mm, thick and increased curvature of lens, short axial length, short diameter, and radius of curvature of the cornea are eyes configuration that more susceptible to develop PACG. Another PCG risk factor are women, older than 40 years old, and has hypermetropia refractive error. Glaucoma history in first degree relatives also one of the risk factors of PACG and suggested of genetic involvement. Recently, retrospective case control research stated that serum triglyceride and uric acid level associated significantly with PACG [2,4].
Classification
Classically, angle closure is divided into 2 main categories: primary angle closure, which is no cause other than anatomical predisposition, and secondary angle closure, which is pathologic cause was identified such as intumescent lens, iris neovascularization, etc. The European Glaucoma Society classified angle closure into: primary angle-closure suspect (PACS), in which the iridotrabecular contact >180o but no evidence of trabecular meshwork or optic nerve damage; primary angle closure (PAC), in which iridotrabecular contact >180o with elevated IOP or PAS but no optic nerve damage; and primary angleclosure glaucoma (PACG), which is characterized by PAS or elevated IOP and glaucomatous optic neuropathy [2,5].
Figure 4: Structure of the normal anterior chamber angle in gonioscopy [6].
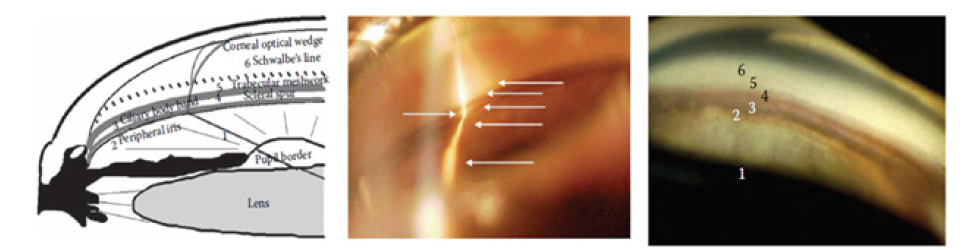
Figure 5: Gonioscopy finding before indentation (above) and after indentation (below) in relative pupillary block [6].
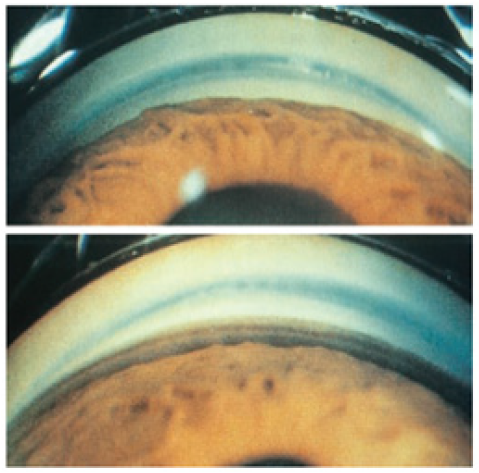
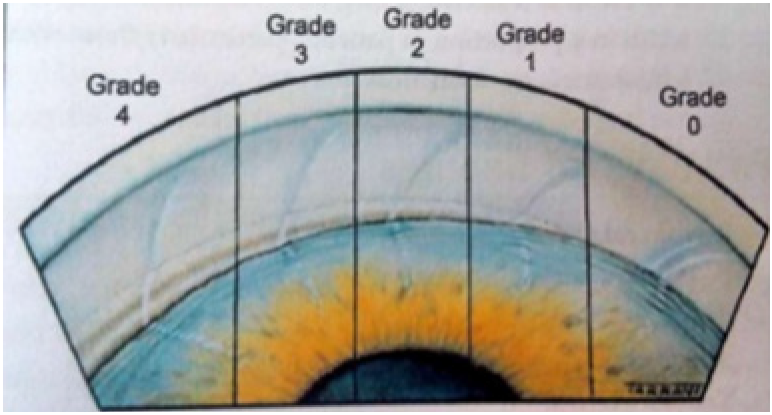
Figure 6: Shaffer modification grading system [7].
Mechanism of angle closure
Mechanism of angle closure focused predominantly at the level of pupil; at the level of the iris and ciliary body; and at the level of the lens. The mechanism at the level of pupil is pupillary block, in which the flow of aqueous humour in impeded between anterior surface of the lens and posterior surface of the iris. The pressure of the posterior chamber becomes high thus push the posterior iris. This results in narrowing of the anger and peripheral bowing of the iris. The pupillary block more common found in mid-dilated pupil. The mechanism at the level of the iris and ciliary body block is a result of anatomy configuration called plateau iris. This configuration defined as thick and anterior inserted iris; and large and posteriorly positioned cilliary body resulting in narrow angle. The mechanism at the level of the lens caused by thicker and more anteriorly positioned lens resulted in shallow anterior chamber and narrow angle [2,5,6].
In other word, angle closure is a result of apposition of the peripheral iris caused by any underlying mechanism (Figure 1-3). This prolong apposition makes the permanent adhesion between peripheral iris and trebecular meshwork, also called peripheral anterior synechiae (PAS). Apposition of the peripheral iris and PAS can be differentiated using indentation gonioscopy [2].
Sign and symptom
Primary angle closure suspect (PACS) defined as narrow anterior chamber angle without elevated IOP, PAS and glaucomatous optic nerve damage. Commonly the patients are asymptomatic, and the clinician get this patient incidentally. Provocative tests such as pharmacologic pupillary dilatation and the darkroom prone position are performed to precipitate angle closure
Primary angle closure (PAC) divided into three subtypes: acute, intermittent and chronic angle closure. In acute angle closure, circumferential iris apposition to the trabecular meshwork leads to rapid and excessive increase in IOP that doesn’t resolve spontaneously (Figure 3-6). In intermittent angle closure, angle closure like acute angle closure but spontaneously resolved. In chronic angle closure, permanent iris apposition the trabecular meshwork resulted in PAS [2,5]. Acute primary angle closure is an ocular emergency which the IOP raises rapidly as a result of relatively sudden blockage of the trabecular meshwork by the iris. Signs and symptoms of acute PAC are ocular pain; headache; blurred vision; halos around lights; nausea; vomiting; high IOP; mid dilated, sluggish and irregular pupil; corneal epithelial edema; congested episcleral and conjunctival blood vessels; shallow peripheral anterior chamber; and mild aqueous flare and cells. The very high IOP may resulted in glaucomatous damage, ischemic nerve damage, retinal vascular occlusion. Prolong iridocorneal contact leads to rapid formation of PAS and may worse the condition. Occlusion of the vessel may result in ischemia of the iris resulted in sector atrophy of the iris, released pigment of the iris and may cause the pupil fixed and dilated. Glaucomflecken may also formed due to necrosis and was seen as small anterior subcapsular opacities. Glaucomflcken is a prove of previous acute PAC [2,5].
Subacute or intermittent angle closure is a condition characterized by episodes of vision, halos, and mild pain caused by elevated IOP. The visual symptoms resolve spontaneously and the IOP may back to normal. Intermittent angle closure may progress to chronic angle closure if not resolve spontaneously. Chronic angle closure may develop after acute angle closure or after intermittent angle closure. Angle closure may also develop gradually and the IOP elevate but the patients are asymptomatic (Figure 6-11). The diagnosis is made based on gonioscopic finding, elevation of IOP and progressive glaucomatous damage.
Figure 7: (a) AS-OCT image captures the anterior segment region or the eye (b) open angle (c) angleclosure [9].

Figure 8: Iris corneal contact in pupillary block viewed by UBM [6].
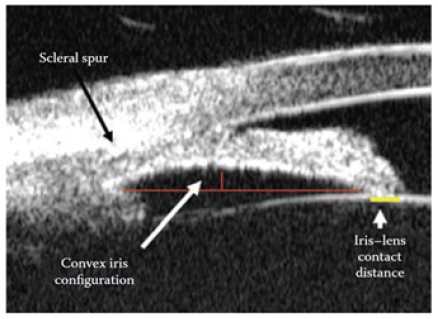
Figure 9: Laser iridotomy [7].
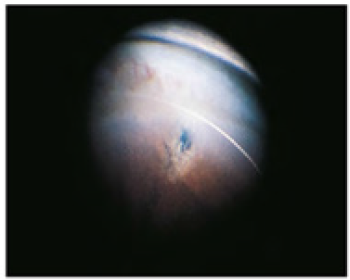
Figure 10: Algorithm for the management of patients with acute angle-closure crisis [11].
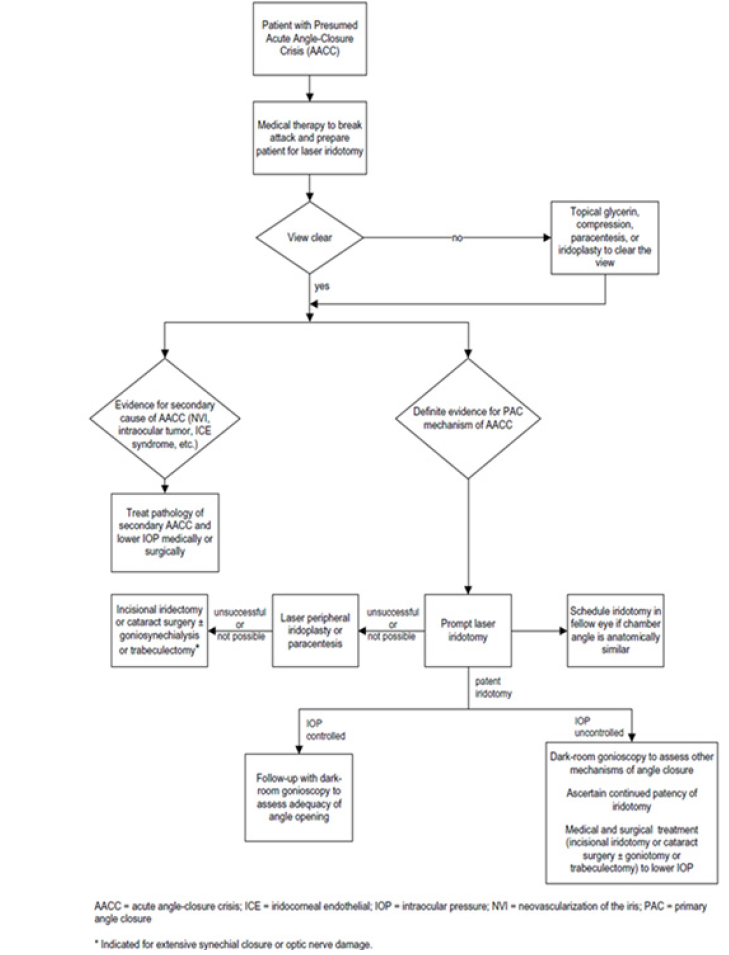
Diagnosis
The diagnosis of primary angle closure glaucoma based on patient history and ophthalmology examination. The patient history, signs and symptoms varied according to progression of the disease as described above. Visual acuity and intraocular pressure should be measured before other examinations. Anterior chamber angle assessment. The examination of the angle is crucial in glaucoma. The assessment of the angle could be done in several methods including van Herick method, gonioscopy and anterior segment imaging techniques. The van Herick method performed in dark room using slit lamp examination. The very slit and bright beam directed 60 degrees from temporal portion of the cornea until anterior chamber barely seen so that depth of the anterior chamber is compared to corneal thickness. Later the anterior chamber is graded from 0 to 4 based on ratio width of empty space (anterior chamber depth) to corneal thickness (grade 0 if no black space between cornea and iris was seen or iridocorneal contact; grade 1if ACD is <¼ corneal thickness and angle closure is likely; grade 2 if ACD is ¼ corneal thickness and angle closure is possible ; grade 3 if ACD is ½ corneal thickness and represents low risk of angle closure; ade grade 4 if ACD is equal to corneal thickness or more and represents very low risk of angle closure). The van Herick methot cannot replace gonioscopy examination [5-7].
Gonioscopy is used to determine the topography of anterior chamber angle using specific goniolens. Dynamic gonioscopy or indentation gonioscopy may be performed to differentiate between appositional iris and PAS. Gonioscopy should be performed in dark room. The patient gets topical anesthesia before examination and later was positioned similar to slit lamp examination. The examiner then put some of the methylcellulose to goniolens in order to fill the empty space between goniolens and patient’s corneal surface. The goniolens put on patient corneal surface and hold the lens during examination. The slit, medium light beam and high magnification is used to assess the 360o anterior chamber angle topography. The landmarks such as Schwalbe line, trabecular meshwork, scleral spur, cilliary body band and peripheral iris are identified. The pathologic findings of angle closure such ae PAS, double hump (found on plateau iris), blood, neovascularization also can be identified during gonioscopy. Then the angle is graded into several available grading systems, but the most commonly used is modified Shaffer grading. Grade 0 was described when no TM could be observed. Grade 1 was recorded when only Schwalbe’s lines and the anterior TM were visible. Grade 2 was recorded when angle structures were visible only until the posterior TM. Grade 3 was considered if all angle structures were visible up to the scleral spur and, finally, grade 4 if all structures were visible up to the iris root and its attachment to the anterior ciliary body [2,5].
Other objective ways to examine the iridocorneal angle (ICA) and for angle grading, include ultrasound biomicroscopy (UBM), anterior segment optical coherence tomography (AS-OCT) and Scheimpflug photography. UBM is essential to evaluate posterior structures to the angle, ciliary body and iris posterior face, but it requires a skilled operator and long image acquisition times. AS-OCT imaging provides clear cross sections of the entire anterior chamber for observation but has limitations when used in clinical management, because of the lack of automated methods to interpret AS-OCT images for the presence of angle-closure. Scheimpflug photography has good reproducibility, but angle image resolution is poor and there is often excessive reflection at the angle. The latest technology for assessment of the anterior chamber angle is automated gonioscopy photography that will capture 360o of anterior chamber angle and will be presented as 64 images [8,9].
Assessment optic nerve head
Assessment of the optic nerve head is crucial to find glaucomatous optic neuropathy findings. This assessment can be done using several methods such as direct ophthalmoscopy, indirect ophthalmoscopy and using silt lamp biomicroscope combined with high magnification posterior pole lens (60D, 78D or 90D0. The latter is more preferable to use because this method provides high magnification, excellent illumination and stereoscopic view of the optic nerve head. Ophthalmoscopic signs of glaucoma are large optic cup, asymmetry of the cups, progressive enlargement of the cup, notching of the rim, vertical elongation of the cup, cupping to the rim margin, nerve fiber layer hemorrhages, nerve fiber layer loss, exposed lamina cribrosa, nasal displacement of the vessels, baring of circumlinear vessels and peripapillary atrophy.
Visual field examination
Visual field examination should be done in every glaucoma patient to assess progression of the disease. The standard method of measuring the visual dysfunction is clinical perimetry. Which measure differential light sensitivity, or the ability of the subject to distinguish a stimulus from a uniform background. Glaucomatous visual field defects are arucuate or Bjerrum scotoma, nasal step, paracentral scotoma, altitudinal defect, general depression and temporal wedge [2].
Treatment
The treatment for PACS can be delayed for asymptomatic patients. Patients are advised to seek medical professional if the symptoms occur such as headache, nausea, vomiting, halo, and blurry vision. Patients also advised to avoid things that can precipitate angle closure such as dim light, pain, emotional upset, fright, several drugs including mydriatic, mitotic, allergy and cold medication, anti-depressant, and some urological drugs. The definitive treatment for PAC and PACG is laser iridotomy. Laser iridotomy using argon or Nd. YAG laser removes some part of peripheral iris thus provide an alternate route for aqueous trapped in posterior chamber to enter the anterior chamber. Despite having laser iridotomy in PAC patients, there is still a chance to develop to PACG especially for patients having appositional anterior chamber more than two quadrants, older age and higher vertical cup disc ratio. For plateau iris, it may be necessary to perform laser peripheral iridoplasty if laser iridotomy was not effective. In LPI, argon laser used to make a stromal burn in peripheral iris to cause contraction and flattening, thereby pulling the iris away from tha globe [10-12].
In acute angle closure laser iridotomy may not be done due to corneal edema. The first thing to do in acute angle closure is to lower the IOP so that laser iridotomy can be performed. The medications that can be given during acute attacks topical cholinergic agents, β-blocker, α2 adrenergic, carbonic anhydrase inhibitor and systemic hiperosmotic agents. Paracintesis also can be performed using 30-gauge needle or blade. Recently, randomized clinical trial proved that combination of dexamethasone subconjunctival injection and conventional therapy can signify accelerate the relief of high IOP.
Conclusion
Primary angle closure glaucoma (PACG), is the second most common form of glaucoma that caused irreversible blindness worldwide. The problem in PACG not only the closure of the anterior chamber angle but also elevated intraocular pressure (IOP). Early diagnosis is very important to prevent blindness due to PACG. The principal management is focusing to open the angle of anterior chamber to prevent IOP elevation.
References
- World Health Organization (2019) World report on vision, Switzerland.
- American academy of ophthalmology (2019-2020) Basic and clinical science course section 10 glaucoma, USA.
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, et al. (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121(11): 2081- 2090.
- Lei Y, Gao Y, Song M, Cao W, Sun X (2020) Retrospective case-control data of serum nitrotyrosine level and clinical biomedical indices in primary glaucoma patients. Data Brief 31: 105706.
- Bagnis A,Traverso CE (2016) Angle Closure Glaucoma. In: Traverso CE, Stalmans I, Topouzis F, Bagnasco L, (Eds.), Glaucoma, ESASO Course Series, Switzerland 8: 38-51.
- Choplin NT, Traverso CE (2014) Atlas of glaucoma. In: (3rd edn), CRC Press, USA.
- Stamper R, Lieberman M, Drake M (2009) Becker-shaffer’s diagnosis and therapy of the glaucomas. In: Stamper RL, Lieberman MF, Drake MV (Eds.), (8th edn), St. Louis: Mosby, USA.
- Teixeira F, Sousa DC, Leal I, Barata A, Neves CM, et al. (2020) Automated gonioscopy photography for iridocorneal angle grading. Eur J Ophthalmol 30(1): 112-118.
- Fu H, Xu Y, Lin S, Kee DW, Baskaran M, et al. (2020) Angle-closure detection in anterior segment OCT based on multilevel deep network. IEEE Trans Cybern 50(7): 3358-3366.
- Qiu L, Yan Y, Wu L (2020) Appositional angle closure and conversion of primary angle closure into glaucoma after laser peripheral iridotomy. Br J Ophthalmol 104(3): 386-391.
- Prum BE, Herndon LW, Moroi SE, Mansberger SL, Stein JD, et al. (2016) Primary angle closure preferred practice pattern® Journal of Ophthalmology 123(1): 1-40.
- Huang W, Li X, Gao K, Zhang X (2020) Combined subconjunctival injection of dexamethasone for the management of acute primary angle closure: A randomised controlled trial. Br J Ophthalmol 104(1): 87-91.
© 2020 Rodiah Rahmawaty Lubis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






