- Submissions

Full Text
Research in Medical & Engineering Sciences
V+ model-a Medical Perspective for Device Development
Daniel van Dort1* and Francien Mels2
1Department of Cardiothoracic Surgery, Netherlands
2Cardiac Boosters, Netherlands
*Corresponding author: Daniël van Dort, Radboudumc, Department of Cardiothoracic Surgery, Geert Grooteplein Zuid, GA Nijmegen
Submission: February 20, 2020 Published: April 09, 2020

ISSN: 2576-8816Volume8 Issue5
Abstract
Only a very small number of innovations in healthcare are ultimately market proof. Most innovations fail because they do not serve an actual market. Different models are available to provide guidance in the development phase of a novel therapy. One of these models is the V model, which was created to align development with stakeholders and to improve efficiency of development cycle. The alignment with stakeholders helps to insure the innovation matches the actual market. The models is very functional in pure technical and digital innovation, however the medical innovation is complex and not fully covered by this model. The novel V+ model introduce an additional layer to the V model, thereby covering all aspects of development in the medical landscape.
Introduction of V+ Model
Ideas for Med Tech concepts are widely available, however, only a very small number of these are ultimately market proof. The main reason for lack of success is the complexity of the total development process, spanning the range from an idea to a reimbursed device. Different models are available to provide guidance in this non-linear and iterative process [1-3]. However, often the intended innovation does not serve an actual market [4].We believe that a dedicated model-focused on stakeholder requirements and validation of these requirements-is essential in order to overcome the mismatch between the projected and the actual market.
One of the available models to structure project management is the V-model [5]. The core of this model consists of the stakeholder requirements, represented on the top left side (Figure 1). The V-model demonstrates the relationships between each part of the development life cycle and its associated phase of testing. The horizontal axis represents project completeness (left-to-right) and the vertical axis represents the level of abstraction (top-to-bottom). All design elements and tests must be traceable to one or more system requirements and every requirement must be addressed by at least one design element and acceptance test. This method is fully in line with the FDA [6].
Figure 1: The V+ model.
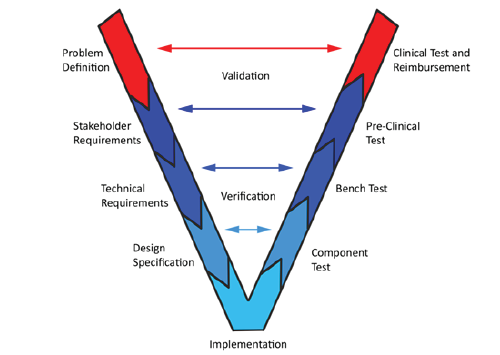
Originally this model was used for software development. In this review we propose a method designed for medical (device) development: the V+ model. The V+ model introduces an additional layer at the input and at the output end of the V Figure 1. On the top-left, or input side, the additional layer is the problem definition. Especially in the medical field where not all (physical) mechanisms are known, thesis a key element for the initiation of an innovation. On top-right side, or output side, of the “V” the clinical approval and reimbursement are added: the working device needs to be applicable to the market with the complex regulatory-and reimbursement systems.
Both the unknown (physiological) mechanism and the complexity of regulatory- and reimbursement systems, makes the medical innovations more prone to failure. Keeping these additional pitfalls in mind during the whole development process, improves the likelihood of success. This article describes the application of the additional elements (i.e. problem definition on the one site and clinical approval and reimbursement on the other site of the V). Furthermore, it details the stakeholder requirements and pre-clinical testing, which are crucial in the Med Tech development and differ from the software development, where the model was originally used.
Problem definition
In the V+ model, the problem definition is the first phase in the development cycle where the problem that needs to be solved needs to be fully understood and the intended use needs to be clearly defined. Both the definition of the problem and the intended use are inputs for the clinical acceptance and reimbursement, which should be considered already at this early stage. In order to fully comprehend the problem in the medical field, we need to take into account different (patho-) physiological mechanisms, anatomical structures, bio-chemical behaviour, indications and contra-indications. Understanding all these aspects is extremely important, since these are important input variables for how the device will be used in the clinical environment. This links directly to the clinical acceptance and reimbursement.
The intended use is not only the starting point for CE and FDA certification, but is also a key input variable for the next development stages. The intended use maybe preventive or curative therapy, improvement of quality of life by reduction of symptoms or by increasing survival. All have a different approach and may have different stakeholders.
Stakeholder requirements
The stakeholder requirements are understood from the stakeholders’ perspective. This phase involves detailed communication with all stakeholders to understand their expectations and exact requirements. This is a very important activity that needs to be carefully managed, as most of the stakeholders are not sure about the exact requirements. The pre-clinical test definition is also started at this stage as stakeholder requirements can be used as an input for pre-clinical testing.
The healthcare sector is a complex landscape of stakeholders with different interests and their own specific requirements. To identify all stakeholders and entities that are involved is important, and one has to bear in mind that in healthcare the user is most often not the buyer nor the ‘end’ customer. This complicates the product development in de medical field, where a clinician looks at usability and effectiveness, the hospital looks at reimbursement of the technology and the insurance company looks at pricing and available – cheaper – alternatives. It is important to understand the needs and expectations of all the different stakeholders in determining the final product [7]. The most common stakeholder groups in the healthcare sector are detailed below.
Central clinical requirements: Risk-benefit ratio
For a therapy to be used it needs to be effective and safe: the effectiveness (benefit) for the patient needs to outweigh the risks of using the product/therapy. When a therapy improves survival, especially when no alternative options are available, the tolerance for complications is much higher (e.g. bone marrow transplants [8]. However, when a therapy is merely supportive or palliative (pain killer [9]), the tolerance for risks is very narrow.
Patient
In all cases where shared decision making is possible, patients will try to find an optimal balance between increased chances of survival, improvement of quality of life, invasiveness, side effects, non-stigmatizing appearance and costs. The latter is very much dependent on the specific local insurance situation of each patient or each patient group.
Medical staff
Medical staff is always looking for the best possible treatment for their patients. The best treatment consists of high effectiveness and low complication rates in combination with the ease of use. Preferably, an innovation needs to fit in the existing workflow. Although medical staff is highly trained, medical errors are still among the most common causes of death in the western world [10]. Therefore, new therapies need to be as straightforward as possible as this increases the uptake and predictability and minimizes errors and complications.
Hospital
Even when doctors and patients are enthusiastic about a novel therapy, it is up to the hospitals’ discretion to use or not to use it. This occurs often in negotiation with the insurance companies, since the latter eventually pay for the novel therapy. The hospital needs to balance the demand of the medical staff with the supply or allowance of the government or insurance company.
Additionally, a hospital needs to take their whole staff into account (technical-, nursing-, IT- and medical staff). All need to be trained to execute or support certain therapies. With the high workload in the current clinical setting and with the increasing risk for burn out of medical staff [11], the prospect of a new therapy resulting in a decreasing workload will be an important factor for therapy adoption.
Insurance company
Healthcare is uniquely organized per region. The decision for an insurance company to reimburse a therapy is complex and often involves a political discussion. In general, for an insurance company a therapy needs to make a significant impact on healthcare against an acceptable cost. A tool to assess the effectiveness of a therapy is the quality adjusted life year, which calculates the effect of a therapy on survival or the quality of life (QUALY) in the same life span. Overall in the western world there is currently an arbitrary standard for how much a QUALY can cost [12].
Pre-clinical testing
Pre-clinical: Pre-clinical (often in-vivo) testing is associated with the stakeholder requirement analysis phase and involves testing of the product in an environment that is as close as possible to the user environment. Pre-clinical tests uncover issues related to the use of the device or therapy in a user environment, which- in view of the multivariable nature of pre-clinical/in-vivo studies-is a critical step in the development. In these studies, it is important to take as many variables into account as possible, without losing focus on the intended use. Experienced research centres and involvement of key opinion leaders who have performed similar studies will help to identify and interpret the important and sometimes unexpected outcomes.
Clinical approval: Clinical approval is associated with the problem definition phase and involves clinical and safety tests, certifications and ultimately guideline acceptance.
Clinical tests: Clinical tests/first in man testing is an important milestone in medical device development. Before starting clinical testing, the novel therapy/device needs to be proven safe and effective on the bench and in pre-clinical testing [13], which is in line with the V+ model. For successful clinical tests, it is important to select an experienced environment and an operator who has experience with the field of interest and preferably has already extensive experience with similar clinical studies. This operator is usually a key stakeholder who has been identified at the start of the project and has been actively involved throughout the whole development process. Furthermore, in de medical field patient selection is critical. This is directly linked to the problem-definition phase, in which the problem was described (identifying e.g. indications and contra-indications) and in which the intended use was defined.
CE-FDA: Regulatory approval is interlinked with the clinical tests as described above. This means that clinical tests cannot start until certain safety tests – defined by the regulatory bodies have been approved – and at the same time positive results of clinical tests are needed for regulatory approval. Regulatory approval is organized per region and is needed in all the countries where the devices are to be used. Since the biggest market medical device market is in de US and Europe, approval by the FDA and CE, respectively, are of most interest for device development. CE and FDA use different classes to indicate the invasiveness of the therapy (From class I to non-invasive to class III long term implantable device). For class II and III devices, the certificates are granted by notified bodies, which assess the conformity of certain products before being placed on the market. The process to obtain regulatory approval is extensive and should be planned as early as possible [14]. Several current developments do not make this process any easier. Currently, in Europe a new Medical Device Directive is being introduced and even the Brexit may have implications for device development, increasing the overall lead time for regulatory approval. At the same time, the FDA is taking steps to improve the implication of new technologies by breakthrough approval decreasing the time to market for new technologies.
Guideline acceptance: Medical decision making is driven by proven effectiveness, with the gold standard being a randomized controlled (preferably double-blinded) study. When therapies or devices are proven to be effective, and superior to standard treatment, or serving an unmet clinical need, they are incorporated in the guidelines. When a therapy makes it to the guidelines the medical staffs is very likely to use that therapy. An example of the guideline for treatment in acute and chronic heart failure can be found in the list of references [15].
Reimbursement: Reimbursement is associated with the problem definition phase and comes after the clinical approval. Medical equipment is notoriously expensive. The pricing is based on an expensive and risky development trajectory and extensive safety procedures. As a result of the very high pricing of medical equipment, typically only reimbursed procedures and devices are used (with exceptions for specific indications). To gain reimbursement there are different challenges ahead, especially for devices [16] and gene- tissue engineering pathways [17]. The most logical pathway is to use an existing reimbursement of a competitive device. More challenging for new therapies is when there are no comparable devices and no reimbursement exists. New reimbursement codes must be allocated by the governing agency. To do so, additional evidence is needed on top of the normal regulatory requirements. In general, reimbursement systems work with a code for a specific therapy or device. For the code there is a certain amount of capital available. These amounts are adjusted according to the market development. For example, insurance companies will look at the cost effectiveness of a therapy and the availability of less expensive alternatives. When the cost effectiveness is lower, the reimbursement decreases accordingly. Different reimbursement systems for each country add another layer of complexity to development in the medical field [18].
Conclusion
The advantage of the V-Model method is that it is very easy to understand and apply. At the same time, the medical field has a complex landscape where (physical) mechanisms should be taken into account as well as the market with the complex regulatoryand reimbursement systems. Therefore, the V+ model has been presented. The V+ model introduces an additional layer to the V model thereby covering the need to take all aspects of the medical landscape into account during the whole development cycle. Figure 1 shows The V+ model. Blue reflects the old V model. On the left side it starts with stakeholders and goes down to implementation. From implementation to the right side is de testing. Red resembles the added medical layer. Where on the left side problem definition precedes the stakeholder requirements. Problem definition includes all defining layers of the medical field, such as anatomy and pathophysiology. On the right side the clinical testing and reimbursement add a layer to the implementation and testing of the innovation. The horizontal part of the V+ model reflects the feedback loop of the verification and validation.
References
- Pietzsch JB, Shluzas LA, Paté C, Yock PG, Linehan JH (2009) Stage-gate process for the development of medical devices. Journal of Medical Devices 3(2).
- Gilman BL, Brewer JE, Kroll MW (2009) Medical device design process. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 3-6.
- Markiewicz K, Van JA, Ijzerman MJ (2014) Medical devices early assessment methods: Systematic literature review. International Journal of Technology Assessment in Health Care 30(2): 137-146.
- Insights C (2019) The Top 20 Reasons Startups Fail.
- Hugh M, Cawley O, Caffcry F, Richardson I, Wang X (2013) An agile V-model for medical device software development to overcome the challenges with plan-driven software development lifecycles, pp. 12-9.
- FDA (1997) FDA Design control guidance for medical device manufacturers.
- Meyer M, Müller I (2006) Networked healthcare: A practical guide to understanding influence networks in the health-care industry. Journal of Medical Marketing 6(4): 250-259.
- Woods WG, Neudorf S, Gold S, Sanders J, Buckley JD, et al. (2001) A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood 97(1): 56-62.
- Sostres C, Gargallo CJ, Lanas A (2013) Non steroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther 15 (Suppl 3): S3-S.
- Makary MA, Daniel M (2016) Medical error-the third leading cause of death in the US. BMJ 353: i21-i39.
- Rothenberger DA (2017) Physician burnout and well-being: A systematic review and framework for action. Dis Colon Rectum 60(6): 567-576.
- Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG (2000) Willingness to pay for a quality-adjusted life year: In search of a standard. Medical Decision Making 20(3): 332-342.
- Agency EM (2017) Strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products. European Medicines Agency, Europe.
- Kaplan AV, Baim DS, Smith JJ, Feigal DA, Simons M, et al. (2004) Medical device development. Circulation 109(25): 3068-3072.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JF, et al. (2016) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 37(27): 2129-2200.
- Raab GG, Parr DH (2006) From Medical Invention to Clinical Practice: The Reimbursement challenge facing new device procedures and technology-part 1: Issues in medical device assessment. Journal of the American College of Radiology 3(9):694-702.
- Abou M, Elsanhoury A, Reinke P (2016) Overcoming challenges facing advanced therapies in the EU market. Cell Stem Cell 19(3): 293-297.
- Boriani G, Burri H, Mantovani LG, Maniadakis N, Leyva F, et al. (2011) Device therapy and hospital reimbursement practices across European countries: a heterogeneous scenario. EP Euro pace 13(suppl_2): ii59-ii65.
© 2020 Daniel van Dort. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






