- Submissions

Full Text
Research in Medical & Engineering Sciences
Determination of The Antimicrobial Sensitivity of Isolated Sea and Mangrove Bacteria in The State of Veracruz, Mexico, Using the Modified E-Test Technique
Ricardo Contreras-Cerón1, José Antonio Rivera-Tapia2, Laura Morales-Lara3 and Rocío Pérez-y-Terrón4*
1Bachelor of Biology, BUAP
2Institute of Sciences, BUAP
3Faculty of Chemical Sciences, BUAP
4Faculty of Biological Sciences, Benemerita Autonomous, University of Puebla, Mexico
*Corresponding author: Rocío Pérez y Terrón, Research Professor, Faculty of Biological Sciences, Autonomous University of Puebla, Puebla, Mexico
Submission: February 03, 2020 Published: February 24, 2020

ISSN: 2576-8816Volume8 Issue4
Abstract
In that study, a total of 22 sea and mangrove samples from the State of Veracruz were isolated and tested for antimicrobial sensitivity with 9 different antibiotics. The objective was to determine the minimum inhibitory concentration using the modified E-test technique. Phenotypic susceptibility tests revealed that the antibiotics with the highest range of action were Levofloxacin with a total of 77% inhibition of total strains, ciprofloxacin with 81%, sulfametazone with 81% and tetracycline was the most antibiotic antimicrobial activity presented with 90%. This provides information on resistance in non-clinical sample bacteria. 3 bacterial species were identified, being: Staphylococcus aureus, Bacillus thuringiensis, Ochrobactrum anthropi.
Keywords: E-test; Resistance; Sensitivity; Mangrove; Sea; Minimum inhibitory concentration; Phenotypic; Strain; Axenic
Abbreviations: MIC: Minimum Inhibitory Concentration; UFC: Colony Forming Units; mm: Millimeters; %: Percentage; RPM: Revolutions Per Minute
Introduction
Bacteria that are insulated in hospitals, fishing farms, sewage, contaminated water mantle, rivers, and lagoons are usually high resistant to antibiotics. Mangroves are a set of trees that inhabit the coast of the sea [1]. These can be tropical or subtropical woody plants that can be found in bays, seas, lagoons and rivers, so they retain a certain amount of salt [2]. The role of microorganisms is crucial in the sea and mangrove for maintenance and development. However, the role played by each of these microorganisms is very little known. In 1928, Alexander Fleming began conducting different experiments using the first antibiotics against gram-positive pathogens, responsible for different diseases, these antibiotics were called Penicillins [3]. Antibiotics are not only used to treat human diseases, but also for the veterinary and livestock area. One problem that arises is the inappropriate use of antimicrobials, in addition many of these compounds are irresponsibly released to the bodies of water, which has created favorable conditions for the appearance and spread of microorganisms with antibiotic resistance [4]. Bacterial resistance is defined as the ability of bacteria to survive in the presence of an antimicrobial, which gives them some advantage in surviving the environment. This type of resistance can be presented as a natural cause [5], or in an acquired way, which occurs in different types of natural habitats, e.g. sewage, seawater, lagoon water, river water, sediments and soils [6]. Antibiotic resistance is known to be a risk to human and environmental health, even at low concentrations [7].
The Epsilometry (E- test) technique is used to determine the minimum inhibitory concentration (MIC) of antibiotics using an antimicrobial concentration gradient [8]. This technique consists of a diffusion in agar for the quantitative determination of susceptibility to microbial agents. After incubation of the plates, an ellipsoidal inhibition zone may be observed. To determine the minimum inhibitory concentration (MIC) shall be the value obtained from the inhibition end with the intersection of the strip [9]. The E-test test has also been used to evaluate activity against mycobacteria, gram-positive and gram-negative bacteria, including Pseudomonas. The objective of this study was to determine the antimicrobial sensitivity of different bacteria seals and mangroves of the Veracruz State in Mexico, using the adapted e-test technique.
There is little information on the current state of the antimicrobial sensitivity of bacteria in the aquifer media, this may be due to bacteria not being in direct or constant contact with humans or an antimicrobial, as many studies are focus on investigating bacteria found in nosocomios such as hospitals, clinics to name a few. Therefore, this work suggests that it is of paramount importance to know the sensitivity or possible resistance to antibiotics of bacteria in this type of habitat, because many people near these areas including tourist areas can acquire a serious infectious disease upon entering such water.
Material and Methods
The water samples of the sea and mangrove of the municipality Chichinit in the state of Veracruz were obtained, they were transported in sterile falcon tubes of 50ml. For the growth of the isolates, the strains were inoculated in Luria Bertani Broth (LB) with water from these sampling sites, in order to take advantage of all the nutrients and minerals that it may contain and that are necessary for the growth of bacteria. The pH of the water was measured with a portable branded potentiometer (HANNA). Once inoculated the strains were incubated in constant agitation at 100 revolutions per minute (RPM) at a temperature of 37 +/- 2 °C. They were sown using the cross-striation method on plates with selective and differential media, in order to have axenic cultures. For the production of anaerobic bacteria, the previous procedure was performed only that at the time of incubation they were placed in a branded anaerobic chamber (BD of BBL). For presumptive identification, colonial morphology was observed, and Gram staining was performed, subsequently the microorganisms were identified by biochemical phenotypic tests [10]. And in some cases, tests such as coagulase and oxidation-fermentation of glucose (OF) were attached. PI 20E and API 20NE galleries (BIOMRIEUX, Marcy I'Etoile-France), were also used following the methodology described by the manufacturer.
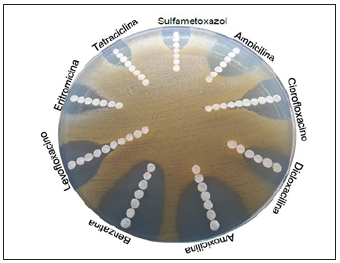
For the determination of antimicrobial sensitivity, 10 microliters of the strains adjusted to a concentration of 1x10-6 CFU/ml, on glass Petri plate (90mm x 15mm) with medium LB, with the mass sowing technique, were sowing 3 discs of filter paper (Figure 1) impregnated with 6 microliters of the antibiotic to be tested were placed. All inhibition halos were measured with a Vernier Digital Brand (TRUPER) in pre-calibrated mm. Parameters were taken as a reference in accordance with the 2015 CLSI 2015 standards. For the determination of the MIC using the E-test, n filter paper discs with progressively diluted antibiotic successive concentrations from 1:10 were used, the highest concentration was located at the edges using glass petri plates (150mm x 30mm) and the center was the lowest concentration (Figure 2). For anaerobic bacteria (90mm x 15mm) plates were used for each antibiotic. After incubation of the plates, an ellipsoidal inhibition zone may be observed. To determine the minimum inhibitory concentration (MIC) shall be the value obtained from the inhibition end with the intersection of the strip
Figure 1:Growth inhibition.

Figure 2: Determination of the MIC using modified E-test technique.
Study area
Location: The municipality Chichinit in the Veracruz State is located between the parallels 20o 09' and 20o 41' of north latitude; meridians 97'06' and 97' 32' west length; altitude between 10 and 300 m. Limits: It adjoins the north with the municipalities of Poza Rica de Hidalgo, Tihuatlán, Cazones de Herrera and the Gulf of Mexico; to the east with the Gulf of Mexico and the municipalities of Revoluta, Gutiérrez Zamora, and Martínez de la Torre; to the south with the municipality of Martínez. Climate: Warm sub humid with rains in summer, medium humidity (40%), warm sub humid with rains in summer, higher humidity (32%) warm wet with abundant rains in summer 28% [11].
Results
A total of 22 strains (19 Mangrove and 3 From Sea) were recovered. The results of Gram staining are presented in Table 1. With API galleries 3 species were identified with 99.9% identity being: Ochrobactrum anthropi, Bacillus thuringiensi) and Staphylococcus aureus. The identity for the other strains was of lower percentage so it is not reported. Sensitivity test results for each of the strains with 9 antibiotics, shown with the diameters of the inhibition halos (Table 2). For bacteria that were sensitive, THE MIC was determined with the modified E-test test for each group of antibiotics (Tables 3-5). Phenotypic susceptibility tests revealed that the antibiotics with the highest range of action were Levofloxacin with a total of 77% inhibition of total strains, ciprofloxacin with and sulfametazone with 81% and tetracycline was the most antibiotic antimicrobial activity presented with 90% (Table 6 & Figures 3-6).
Figure 3: Diameter of the halos inhibiting the strains for Ciprofloxacin.
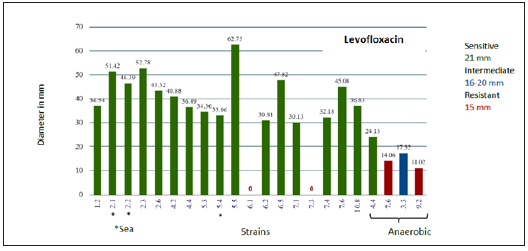
Figure 4: Diameter of the halos inhibiting the strains for Ciprofloxacin.
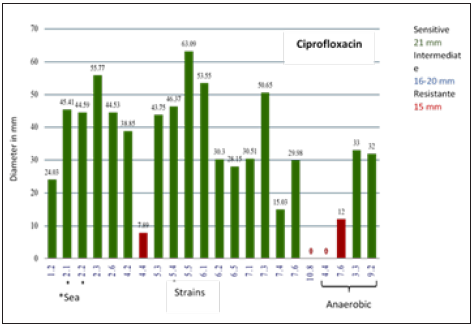
Figure 5: Diameter of the halos inhibiting the strains for Sulfamethoxazole+ Trimethoprim.

Figure 6: Diameter of the halos inhibiting the strains for Tetracycline.
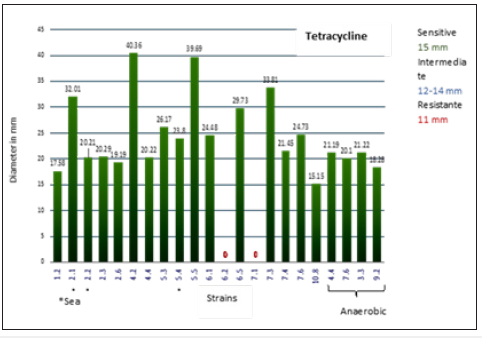
Table 1: Morphological identification of Gram-stained strains.
Table 2: Diameters of inhibition halos for nine antibiotics.
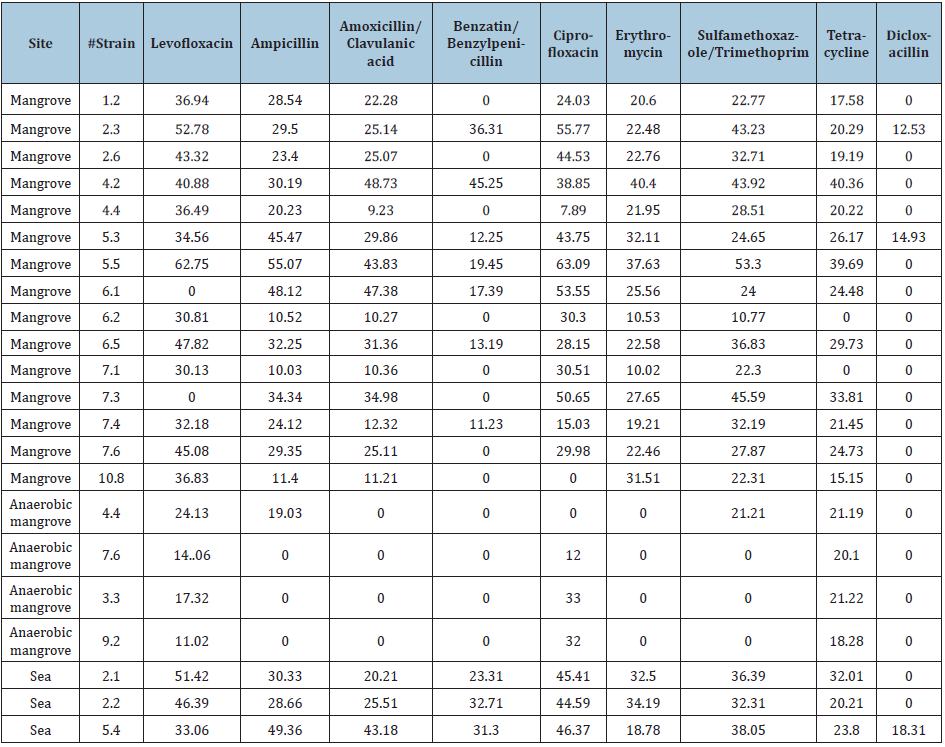
Table 3: Determination of the MIC for the quinolone group.
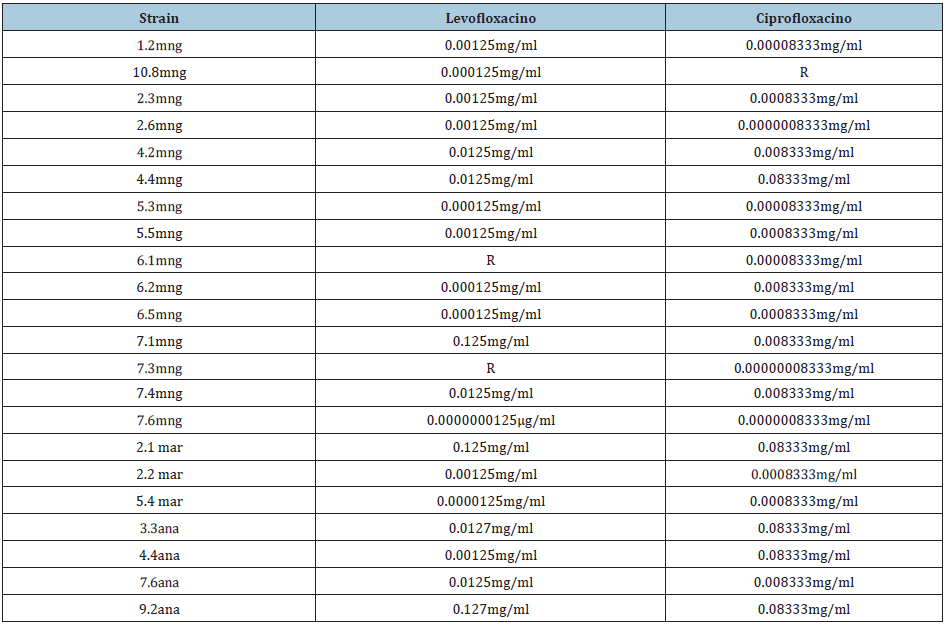
R: Resistant *All measurements are given in millimeters.
Table 4: Determination of the MIC for the beta-lactam group
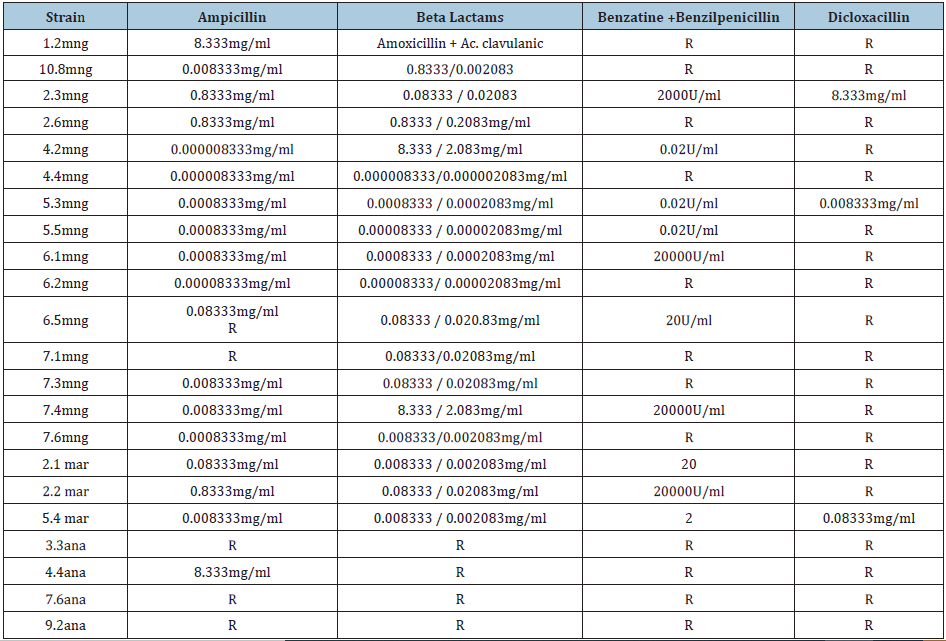
Table 5: Determination of MIC for macrolides, tertracyclines and sulfonamides.

R: Resistant
Table 6: Percentage of antibiotic sensitivity and resistance.

Discussion
Levofloxacin and ciprofloxacin, which had a high inhibition action, are found in the quinolone group. They act by interfering with bacterial DNA by binding topoisomerase 2 and 4, enzymes important for super-rolling the double DNA helix [12]. In the DNA chain, quinolones inhibit the ligase activity of the toposoimerase. The DNA-girasa enzyme cuts the double helix of the chromosomal DNA into small fragments and over-rolls in the opposite direction to begin sealing the ends that were cut. Quinolones prevent the closure of the rupture ends, the main quinolones also include, Ofloxacin, Moxifloxacin and Norflaxino [13]). Sulfametazone is found in the group of sulfonamides that act by inhibiting the incorporation of PABA (para-aminobenzoic acid) for the formation of folic acid, hence its selective antibacterial effect [14]. Tetracyclines that had the highest level of inhibition are antibiotics that work by inhibiting protein synthesis at the ribosome level. In subunit 30 S act the Tetracyclines and on subunit 50 S act chloramphenicol, lincosamines and in both subunits aminoglycosides, all acting in interfering with the fixation of the NRNA and the mRNA codon so that there is no full reading and cannot be synthesize the amino acids that form proteins. Some of the members of tetracyclines are: Tetracycline, Tigecycline, Minocycline. In the case of the family of aminoglycosides comprises: Streptomycin, Kanamycin, Tobramycin, Gentamicin [15].
Today antibiotic resistance is increasingly increasing, this means that treatments for many diseases are obsolete and inefficient, this problem not only arises in the nosocomios, but has spread in other environments such as water, food, agriculture and livestock. According to the work of Holguin et al. [16], 2011 [16], in the mangrove of Balandra, La Paz. mentions that they isolated bacteria from the genera Aeromonas, Arthrobacter, Azospirillum, Azotobacter, Clostridium, Corynebacterium, Rhizobium, Klebsiella, Paracoccus, Phyllobacterium, Oceanomonas and Vibrio, being important for nitrogen fixation in nitrogen marine environments. otherwise in our work no bacteria associated with the work of the aforementioned author were found, this may be due to the site of where they were isolated and the degree of contamination it may have. Maeda et al. [17] in the mangrove of Nakama-gawa, Japan, report that Bacillus thuringiensis is a microbiota bacterium common in sea soils, freshwater and marine sediments, in this work this species was managed to isolate this species since as mentioned by the author is common find it in these kinds of environments.
In the work of Contreras et al. [18] they mention that the genus Ochrobactrum is resistant to beta-lactams and for this reason it is difficult to treat infectiously this bacteria. In our work we found that this genus was sensitive to Benzatine, Ampicillin, Amoxicillin with the exception of Dicloxacillin, which if it exhibited resistance. It should be noted that the sensitivity of this strain may be due to the place of origin, since as it was oisseist from the mangrove it does not present a higher degree of resistance compared to a strain of clinical origin. In works such as Berglund [19] quinolone-resistant bacteria have been found due to the presence of resistance genes such as qnrA in the plasmid pMG252, present in isolated strains in different environments in countries such as France, Turkey, Germany, Switzerland and Mexico. In this work because Bacillus thuringiensis and strain 4.4 were resistant to ciprofloxacin and 6.1 and 7.3 strains with resistance to levofloxacin, could be due to a possible gene already reported as the qnr gene that gives resistance to quinolones.
Conclusion
Microorganisms could be axenicly isolated in nutrient-like means for identification, antibiotics with higher inhibitory range were levofloxacin and tetracycline, while antibiotics with lower spectrum of action were dicloxacillin and benzatin.
Acknowledgement
This research was conducted with the support of the Vice- Chancellorof Research and Postgraduate Studies of the Benemerita Autonomous University of Puebla, Mexico.
References
- Hoff R, Hensel P, Proffitt EC, Delgado P (2002) Oil spills in mangroves. national oceanic and atmospheric administration, pp. 7-69.
- Kathiresan Ky, Qasim SZ (2005) Biodiversity of mangrove ecosystems. Hindustan Publishing Corporation. New Delhi, India.
- Tan SY, Tatsumura Y (2015) Alexander fleming (1881-1955): Discoverer of penicillin. Singapore Medical Journal, 56(7): 366-367.
- Zhang X, Zhang T, Fang H (2009) Antibiotic resistance genes in water environment. Appl Microbiol Biotechnol 82(3): 397-414.
- Séveno N, Kallifidas D, Smalla K, Elsas J, Collard J, et al. (2002) Occurrence and reservoirs of antibiotic resistance genes in the environment. Reviews in Medical Microbiology 13(1): 15-27.
- Kruse H, Sorum H (1994) Transfer of multiple drug resistance plasmids between bacteria of diverse origins in natural microenvironments. American Society for Microbiology 60(11): 4015-4021.
- Kümmerer K (2009) Antibiotics in the aquatic environment-a review-part II. Chemosphere. Germany 75(4): 435-441.
- Vjera V (2002) Evaluation and indication of diffusion-dilution techniques (epsilometry). Chilean Journal of Infectology 19(Supl 2): 85-87.
- Picazo J (2000) Basic Methods for the study of antimicrobial sensitivity. Seems, p. 54.
- MacFaddin JF (2003) Biochemical tests for the identification of bacteria of clinical importance. In: 3rd (edn), Editorial Panamericana Medica. Madrid. Spain. pp. 92-97, 206-216, 223-235,306-311, 397-410.
- Government of the State of Veracruz (2016) Mexico.
- Alós, JI (2009) Quinolones infectious diseases and clinical microbiology. The Sevier Microbiology Service, pp. 290-297.
- Walsh C (2003) Antibiotics, actions, origins, resistance. ASM Press, pp. 3-5.
- Paredes F, Roca J (2004) Antibiotic action, antimicrobial measurement perspective. Pharmaceutical Scope 23(3).
- Palomino J, Pachón J (2002) Aminoglycosides. The Sevier, pp. 105-115.
- Holguin G, Vázquez P, Sánchez J, López, Y, Flores A (2011) Mangrove microbiology. Research gate Magazine, Mexico.
- Maeda M, Mizuki E, Hara M, Tanaka R, Akao T, et al. (2001) Insolation of Bacillus thuringiensis from intertidal blackish sediments in mangroves. Japan. Microbiol Res 156(2): 195-198.
- Contreras R, Majalca C, Aguilera MG (2018) Ochrobactrum anthropi. Chilean Journal of Infectology 35(4): 431-432.
- Berglund B (2015) Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infection Ecology & Epidemiology 8(5): 28564.
© 2020 Rocío Pérez y Terrón. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






