- Submissions

Full Text
Research & Investigations in Sports Medicine
The Effect of Active Vitamin D on Diabetic Nephropathy A Meta-Analysis of Randomized Controlled Trials
Xin Mou*, Di Yi Zhou and Dan Yang Zhou
Department of Endocrinology, China
*Corresponding author:Xin Mou, Department of Endocrinology, China
Submission: November 20, 2018; Published: March 22, 2019

ISSN: 2577-1914 Volume4 Issue5
Abstract
Objective: The system evaluation of clinical curative effect of different concentration active vitamin D for diabetic nephropathy.
Methods: We searched the Chinese VIP, Wan Fang Data, China National Knowledge Infrastructure, PubMed, Embase, and Google Scholar databases for relevant studies and extracted all eligible data. RevMan5.3 software was used for statistical analysis.
Result: 20 randomized controlled trials (n=1450) met our criteria and were used for data extraction. The treatment groups have 856 cases and the control groups have 594 cases. The results of statistical analysis show that compared with the group of using ACEI/ARB simply, the combination of ACEI/ARB and different doses active vitamin d (0.25ug/d, 0.50ug/d, 0.25-0.5ug/d)can significantly reduce the 24- hour urinary protein (0.46%, P<0.00001) and (0.22%, P < 0.00001), and can decrease the hypersensitive C-reactive protein (0.63%, P=0.001) and (0.6%, P < 0.00001).However, there was no obvious difference in glycosylated hemoglobin and glomerular filtration rate, lower serum creatinine, increase calcium ion concentration.
Conclusion: Meta-analysis showed that compared with the group of using ACEI/ARB simply, the impact of active vitamin D on diabetic nephropathy obviously, Especially 24-hour urine protein and hypersensitivity C-reactive protein. more randomized controlled trials are needed to be confirmed.
Keywords: Active vitamin; Diabetic; Nephropathy; Meta-analysis
Introduction
Diabetic Nephropathy (DN) is one of the most common microvascular complications of diabetes mellitus (DM). Many patients with DM are predisposed to developing diabetic nephropathy and die from it. In 2007,KDOQI (Kidney Disease: Improving Global Outcomes) proposed “diabetic nephropathy” should be replaced by “Diabetic Kidney Disease(DKD)”. In recent years, the incidence of diabetes and diabetic nephropathy are on the rise. Both type 1 and type 2 diabetes, 30%-40% of the patients suffered kidney damage, in type 2 diabetes, there will be about 5% of the patients were diagnosed with diabetes while existed diabetic kidney damage [1]. In the face of such a large group, so preventing the happening of the diabetic nephropathy and postponing the progress of DN are pressing issues. At present, Chinese guideline for type 2 diabetes pointed out that in addition to diet, lifestyle changes, control blood glucose and blood pressure and correcting dyslipidemia, and end-stage kidney transplants and dialysis treatments, and angiotensin converting enzyme inhibitors (ACEI) and angiotensin II receptor antagonist (ARB), these drugs can effectively block renin- angiotensinaldosterone system, reduce urinary albumin, delay the damage of kidney.
Vitamin D can be obtained from food, and also can be approved by 7- dehydrogenation of cholesterol in the skin of the transformation of ultraviolet irradiation. Many studies have confirmed that vitamin D deficiency is associated with many diseases [2], especially in the kidney disease and the most representative is diabetic nephropathy patients [3,4] and the lower vitamin D levels will lead to heavier diseases. Animal experiments showed that the vitamin D deficiency can increase the activity of RASS system [5]. In addition, the lower vitamin D levels will be more likely to insulin resistance, the more obvious [6]. In recent years, there are a lot of studies on the basis of ACEI or ARB therapy using active vitamin D, to reduce the proteinuria and protect the kidney. Therefore, in order to confirm the treatment with active vitamin D for DKD is effective, this study will be published the activity of Vitamin D in the treatment of diabetic nephropathy system evaluation on the randomized controlled trials.
Methods
This review was conducted and reported according to the preferred reporting items for systematic reviews and meta-analysis statement issued in 2009 (Checklist S1).
Literature searching
A literature search was conducted using the Chinese VIP, wan fang data, china national knowledge infrastructure, Pubmed, Embase, manual retrieval of reference documents, and google scholar databases for randomized controlled trials by using the keywords “Active Vitamin D” and “Diabetic Nephropathy” through December 2017. There were no language preferences.
Inclusion and exclusion criteria
Eligible studies included in this meta-analysis met the following criteria:
A. Patients with diabetic nephropathy were enrolled
B. The study included at least two case control groups: ACEI or ARB therapy and Active vitamin D with ACEI and ARB therapy
C. 24 hours urine protein levels, glycosylated hemoglobin, serum creatinine, hypersensitive C-reactive protein, blood calcium ion concentration and GFR were reported in the study
Exclusion criteria included:
A. Research for the case-control studies, reviews, case reports, reviews, repetition, retrospective study
B. No control groups
C. Unable to get literature full-text, outcome indicators are not consistent
D. Studies conducted in animal models (mice, rats, rabbits, and others)
E. Sample size is too small (<10)
F. Follow-up time is less than 4 weeks of clinical research
G. A definitive diagnosis for the other diabetic nephropathy patients with chronic kidney disease
H. The quality of the literature is too low and is not included in the analysis
Data extraction and quality of literature evaluation
Two reviewers independently extracted the data using a special data extraction form. The following information was recorded:
A. First author’s name and publication year
B. Average age of patients
C. Sample size of each group
D. Treatment strategies, follow-up time (weeks) and adverse reactions of each group
E. The discrete values of various indicators. Disagreements were resolved by the intervention of a third reviewer.
According to Cochrane risk of bias assessment tools [7] into the research of random distribution method, the hidden and blind method, data integrity, selective reports the results of the study, and other such as bias for quality evaluation. For each included in the study, in view of the above article 6 a “yes” (low bias) and “No” (high bias), “Not clear” (the lack of relevant information or bias is not sure) [8].
Outcomes and Statistical Analysis
The Cochrane collaboration RevMan5.3 statistical software for meta- analysis. the standard deviation (SMD) or mean deviation (MD) and 95% confidence intervals (CI) for the outcome of continuous variables were determined. The value of I2 was assessed for heterogeneity, When the statistical heterogeneity between studies (P<0.1, I2<50%), by using the random effects model metaanalysis; When P>=0.1, I2>=50%, by using the fixed effects model for meta-analysis.
Result
Study selection and characteristics
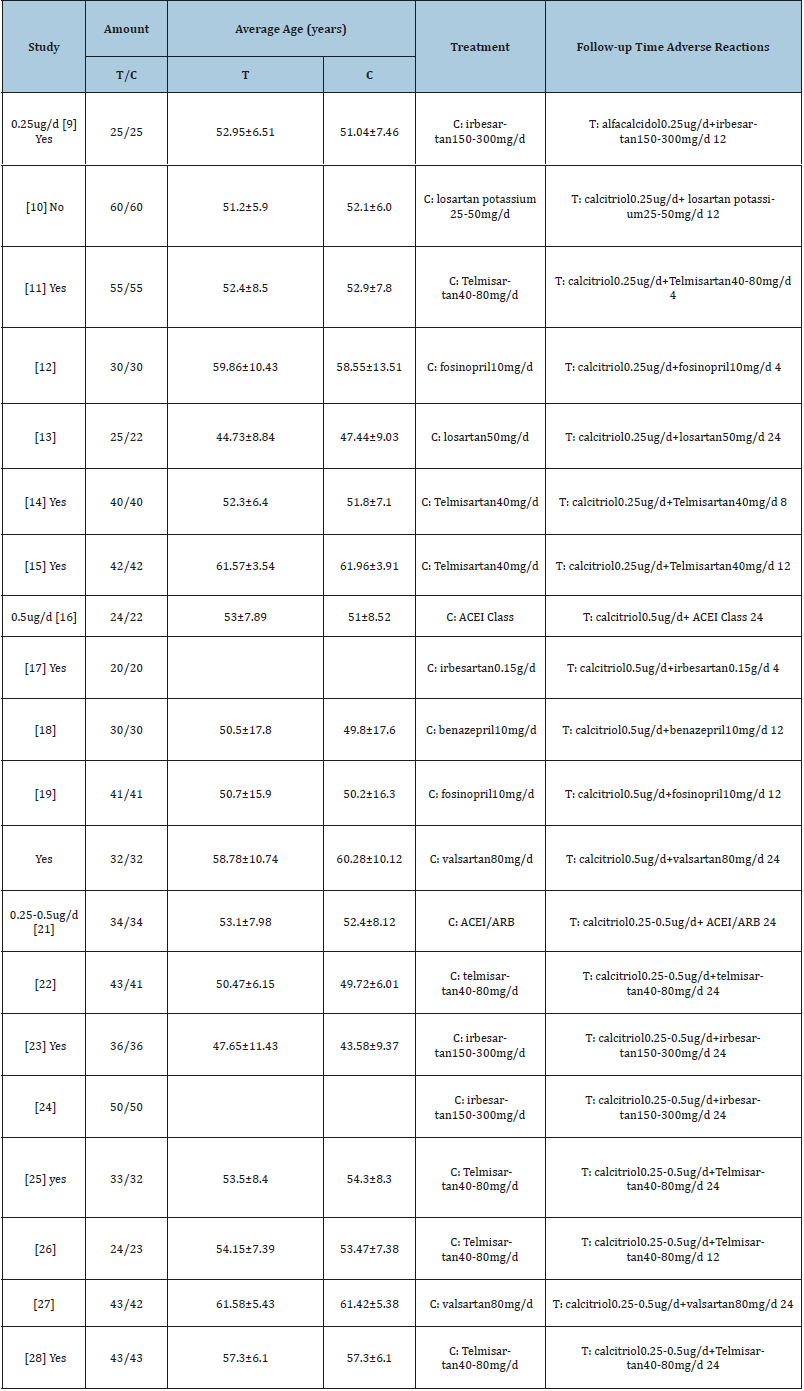
As shown in the flow diagram in (Figure 1), A total of 558 articles were obtained from the initial database search. After perusing the title and abstracts, 59 randomized controlled trials deemed relevant for active vitamin D in diabetic nephropathy patients therapy reviewing. After excluding duplicates, 43 articles were fully read. Of these, 20 met the inclusion criterion, all of which involved studies conducted in China. Data from the included articles were extracted and are shown in (Table 1). T as the treatment group, C as the control group (Figure 1 & 2); (Table 1).
Table 1:Characteristics of included studies.
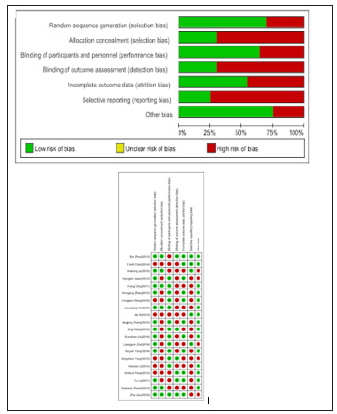
Active vitamin D effects on 24-hour urine protein
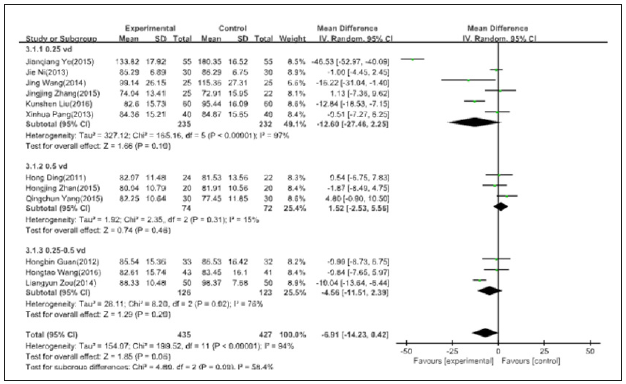
18 studies were included in the meta-analysis, in which the dosage of 0.25ug/d of active vitamin D has six papers, due to statistical heterogeneity between data, and by using the random effects model meta-analysis Dose of 0.5ug/d of active vitamin D has three papers, because the sample quantity is too little, heterogeneity is too big, not be analyzed; Dose of 0.25 to 0.5ug/d of active vitamin D has nine studies, with no statistical heterogeneity between data, a meta-analysis using fixed effect model. 0.25ug/d and 0.25 to 0. 5ug/d respectively shows: he treatment group of 24 hours urinary protein quantity is lower than control group [MD=-0.46, 95%, CI=(- 0.57, -0.34)], [MD=-0.22, 95%, CI=(-0.32, -0.12)], the results have statistical significance (Figure 3).
Figure 1:Search flow diagram for retrieving relevant studies.
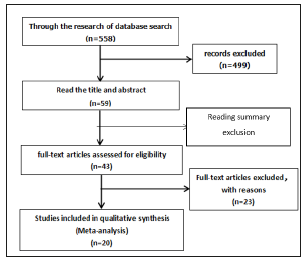
Figure 2:The quality evaluation of included studies..
The influence of active Vitamin D of HbA1c
Eight articles were incorporated into which the dose of 0.25ug/d active vitamin D has three papers, statistical heterogeneity between data, by using the random effects model meta-analysis. Dose of 0.5ug/d active vitamin D has only one article, because the sample quantity is too little, heterogeneity is too big, not be analyzed; Dose of 0.25-0.25ug/d active vitamin D has four articles, because of the heterogeneity between data statistics, a meta-analysis using random effects model 0.25ug/d and 0.25-0.5ug/d respectively shows: [MD=0.01, 95%CI = (-0.18, 0.2)], [MD=0.01, 95%CI =(-0.11, 0.14)], there is no statistical significance (Figure 4).
Figure 3:VD’s influence on the 24 hours urinary protein quantitative (g/24h)..
Figure 4:0.25ug/d VD to the effects of HbA1c.
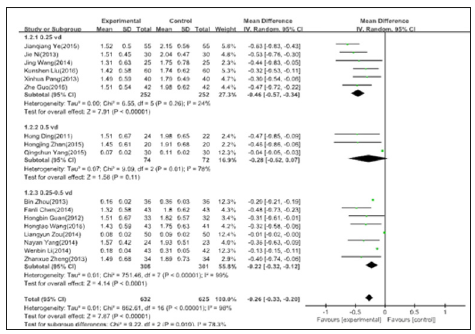
The influence of active vitamin D on serum creatinine
Be included in a total of 12 studies, of which the dose of 0.25ug/d of active vitamin D has six papers, no statistical heterogeneity between data, using fixed effect model to meta-analysis; Dose of 0.5ug/d has three piece of active vitamin D, heterogeneity between data statistics, a meta-analysis using random effects model; Dose of 0.25-0.5ug/d active vitamin D has three papers , with no statistical heterogeneity between data, a meta-analysis using random effect model .0.25ug/d,0.5ug/d and 0.25-0.5ug/d respectively shows: [MD=-9.32, 95% CI = (-11.69, -6.96)], [MD =1.52, 95% CI=(-2.53, 5.56)], [MD=-7.01, 95% CI=(-9.95,-4.07)], the dose of 0.5ug/d group heterogeneity is too big, which should be removed as a result, there is statistical significance (Figure 5).
Figure 5:VD effects on serum creatinine..
The influence of active vitamin D in the HS-CRP
Seven articles were included in the meta-analysis, in which the dosage is 0.25ug/d active vitamin D has two papers, with statistical heterogeneity between data, using random effect model to meta-analysis; Dose of 0.5ug/d of active vitamin D has two studies, heterogeneity between data statistics, a meta-analysis using random effects model; Dose of 0.25 -0.5ug/d active vitamin D has three papers, heterogeneity between data statistics, a metaanalysis using random effects model .0.25ug/d, 0.5ug/d and 0.25- 0.5ug/d respectively shows: [MD=-8.37, 95% CI=(-25.11, 8.37)], [MD=-0.63, 95% CI = (-1.01, -0.24)], [MD=-0.6, 95% CI=(-0.84, -0.35)], the results have statistical significance (Figure 6).
Figure 6:VD effects of HS-CRP.

Active Vitamin D’s impact on calcium ion concentration
11 articles were included in the meta-analysis, in which the dosage of 0.25ug/d has three piece of active vitamin D, statistical heterogeneity between data, by using the random effects model meta-analysis; Dose of 0.5ug/d of active vitamin D has four chapters, with no statistical heterogeneity between data, a meta-analysis using fixed effect model; Dose of 0.25-0.25ug/d of active vitamin D has four chapters, with no statistical heterogeneity between data, a meta-analysis using fixed effect model .0.25ug/d, 0.5ug/d and 0.25- 0.5ug/d respectively shows: [MD=0.05, 95% CI=(-0.02, 0.11)], the results have no statistical significance;[MD=0.29, 95% CI=(-0.27 0.86)], the result was statistically significant;[MD=0.01, 95% CI=(- 0.06, 0.07)], the results have statistical significance. Results three groups (Figure 6) [MD=0.12, 95% CI= (-0.07, 0.31)], the result was not significant (Figure 7).
Figure 7:The influence of active vitamin D for GFR.

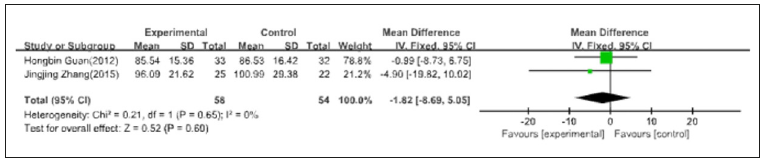
A total of 2 articles were included in this meta-analysis, in which the dosage of 0.25ug/d one article of the active vitamin D and the dose of 0.25-0.25ug/d one article of the active vitamin D, no statistical heterogeneity between data, by using the fixed effects model metaanalysis, the results showed [MD=-1.82, 95% CI=(-8.69, 5.05)], the result has no statistical significance (Figure 8).
Figure 8:The influence of active Vitamin D for GFR.
Discussion
This system evaluation into 20 randomized controlled trials (n=1450), the treatment group has 856 cases, and the control group has 594 cases [9-28]. Active vitamin D combined ACEI/ARB than merely using ACEI/ARB drugs in 24 hours urinary protein, serum creatinine, blood calcium ion concentration and hypersensitive C-reactive protein has obvious curative effect, and according to different experiment chooses the dose of vitamin D is divided into three groups, further standardize the scope of variables and makes the evaluation more scientific and reliability. Diabetic nephropathy is a common one of the serious complications of diabetes, its main pathological features of renal hypertrophy, renal tubular basement membrane thickening, and glomerular mesangial cell proliferation and renal tubular mesangial area increased extracellular matrix, leading to renal function impairment, glomerular fibrosis [29].
At present, the role for Vitamin D mechanism of diabetic nephropathy are mainly inhibiting renin- angiotensin-aldosterone system, inhibition of transforming growth factor β, inhibiting inflammatory cytokines, regulating immune response and protection of Sertoli cell. Active Vitamin D3 can be an increase in intracellular calcium ion concentration, activate the islet B cells secrete insulin [30], the immunoregulatory effects by inhibition of pancreatic islet B cells apoptosis [31], which play the role of protecting islets of course, excessive intake of Vitamin D can lead to VD poisoning, mainly with hypercalcemia, urine with thirst, nausea, vomiting, and constipation, and even life-threatening, so when using Vitamin D or active Vitamin D, should be tested periodically blood 25 (OH) D and urinary calcium levels.
Still exist deficiencies in this study, relevant randomised controlled trials are limited, sample size is small, the quality of the literatures is not high, and the follow-up time is short, and observation of the index is not comprehensive. so, we still need a lot of studies to confirm the effect of active vitamin D on diabetic nephropathy. In conclusion, our study is the first meta-analysis to evaluate the relationship between Different dose of active vitamin d and diabetic nephropathy.
References
- Jianying N, Yong G (2008) Diabetic kidney disease diagnosis and treatment guidelines. Chinese Journal of Practical Internal Medicine 28(10): 833-834.
- Zehnder D, Landray MJ, Wheeler DC, Fraser W, Blackwell L, et al. (2007) Cross-sectional analysis of abnormalities of mineral homeostasis, vitamin D and parathyroid hormone in a cohort of pre-dialysis patients. The chronic renal impairment in Birmingham (CRIB)study. Nephron Clin Pract 107(3): C109-C116.
- Doorenbos CR, Van den BJ, Navis G, De Borst MH (2009) Possible renoprotection by Vitamin D in chronic renal disease: Beyond mineral metabolism. Nat Rev Nephrol 5(12): 691-700.
- Ishimura E, Nishizawa Y, Inaba M, Matsumoto N, Emoto M, et al. (1991) Serum levels of 1, 25-Dihydroxy Vitamin D, 24, 25-dihydroxyvitamin D and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int 55(3): 1019-1027.
- Cheng Q, Boucher BJ, Leung PS (2013) Modulation of hypovitaminosis Dinduced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet rennin-angiotensin system in mice. Diabetologia 56(3): 553-562.
- Scragg R, Sowers M, Bell C (2004) Serum 25-Hydroxyvitamin D, diabetes and ethnicity in the third national health and nutrition examination survey. Diabetes Care 27(12): 2813-2818.
- Higgins JPT, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions, UK.
- Jing Li, Youping L (2008) Improving and devoloping cochrane systematic review. Chin J Evid based Med 8(9): 742-743.
- Jing W, Baochao C (2014) Clinical Observation on 1,25-dihydroxyvitamin D3 and irbesartan treatment of diabetic nephropathy. International Journal of Urology and Nephrology 34(1): 58-60.
- Kunshen L (2016) Clinical study on calcitriol soft capsules combined with losartan in treatment of diabetic nephropathy. World Phytomedicines 31(7): 1024-1027.
- Jianqiang Ye, Shunbin Li, Guorong Z (2015) Clinical observation of calcitriol soft capsules combined with telmisartan tablets in the treatment of early diabetic nephropathy. China Pharmacy 26(18): 2470- 2472.
- Jie Ni, Peng Xu, Jun Z (2013) Clinical observation of the therapeutic effect of calcitriol combined with fosinopril on type 2 diabetic nephropathy. Pract Geriatr 27(2): 119-122.
- Jingjing Z, Xiuyan W, Li Hao (2015) Effect of calcitriol combined with losartan on diabetic nephropathy and influence on pulse wave velocity and ankle brachial index. Chin J Prim Med Pharm 22(4): 538-541.
- Xinhua P (2013) Observation of clinical effects of calcitriol combined with telmisartan on diabetic nephropathy. China modern doctor 51(20): 52-54.
- Zhe Guo, Yusha W (2016) Effect analysis and application of three diabetic nephropathy reported ossification alcohol combined with telmisartan treatment. China Health Care & Nutrition 3(5): 287-287.
- Hong D, Chuan Z, Zongqian W (2011) Calcitriol proteinuria and highsensitivity c-reactive protein in patients with diabetic nephropathy. Shandong Medical Journal 51(41): 80-81.
- Hongjing Z, Shan K, Qiuxia C (2015) Effect observation of calcitriol assisted treatment for diabetic nephropathy. China modern medicine 22(17): 123-128.
- Qingchun Y, Chunling G, Ping H (2015) Clinical observation on the treatment of early diabetic nephropathy with three alcohol and Benner Pury. Heilongjiang medical journal 39(9): 1040-1041.
- Yu Lu, Manyan Z (2011) The effect of three combined with Phu Simpson Leigh on blood and urine MCP-1 in patients with type 2 diabetic nephropathy. Chin J Mod Drug App 5(5): 155-156.
- Hailong Y (2016) Effect of calcitriol combined with valsartan in treating diabetic nephropathy. Journal of Jilin University, China, pp. 1-34.
- Xuezhan Z, Yihong X (2013) Clinical observation on the treatment of diabetic nephropathy with three alcohol. Strait Pharmaceutical Journal 25(9): 133-134.
- Hongtao W, Yuanli H (2016) The clinical curative effect of Calcitriol Soft Capsules and Telmisartan Tablets in the treatment of early diabetic nephropathy. Anhui Medical and Pharmaceutical Journal 24(4): 790- 792.
- Bin Z, Yan Z (2013) Curative effect of calcitriol combined with Irbesartan in the treatment of diabetic nephropathy. China Medical Herald 10(24): 86-91.
- Yunliang Z (2014) The effect analysis of calcitriol combined with irbesartan in the treatment of diabetic nephropathy. Mod diagn treat 25(21): 4803-4806.
- Hongbin G, Hua H, Wenmu H (2012) Effect of treatment of diabetic nephropathy with ossification in three alcohol combined with telmisartan. Guangdong Medical Journal 33(16): 2488-2490.
- Yanna Y, Yubao Z, Jie F (2014) The clinical effect of calcitriol combined with telmisartan in the treatment of diabetic nephropathy. China Modern Medicine 21(4): 57-61.
- Wenbin L (2014) Therapeutic effect of three combined with valsartan on diabetic nephropathy. Medical Information 27(1): 364-365.
- Fanli C (2014) Analysis of ossification in three alcohol and the effect of telmisartan combined with the treatment of diabetic nephropathy. Guide of China Medicine 12(14): 219-220.
- Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, et al. (2008) Diabetetic nephropathy: Mechanisms of renal disease progression. Exp Biol Med 233(1): 4-11.
- Rabinovitch A, Suarez PWL, Sooy K, Strynadka K, Christakos S (2001) Expression of calbindin-D(28k) in a pancreatic islet beta-cell line protects against cytokine-induced apoptosis and necrosis. Endocrinology 142(8): 3649-3655.
- Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L (2002) A 1alpha,25-Dihydroxyvitamin D (3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51(5): 1367- 1374.
© 2019 Xin Mou. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






