- Submissions

Full Text
Research & Development in Material Science
Irradiation Technology
Stanislav Ordin*
Russian Academy of Sciences, Russia
*Corresponding author:Stanislav Ordin, Russian Academy of Sciences, Russia
Submission: September 01, 2025;Published: September 18, 2025

ISSN: 2576-8840 Volume 22 Issue 3
Abstract
Using Private (Local) Regularities and Abstract (Schizophrenic) Models, one can only build an imitation of the General Picture of Nature. So, without using the Fundamental Laws, we are doomed to only imitate progress, moving towards the goal as inanimate Brownian particles, and wasting the unreasonable movement of Life on this. And to create fundamentally new materials when reaching the limits of materials used by technology, it is necessary to use a new factor that increases the probability of obtaining new properties of materials. Such a factor can be the use of resonant X-ray irradiation, with the help of which it is possible to achieve a new Chemistry - Chemistry based on the interaction of the internal electron shells of atoms.
Keywords:Adiabatic decomposition of energy; The order of forces due to the external electron shells; Ionization Potentials; Extreme characteristics of materials
Introduction
The creation of modern materials is, in principle, determined by how far we can deviate from Normal (Earthly) Conditions. In fact, we are going through the chemical variants that the Earth’s surface has once gone through [1]. And there are few cases when a material has been created that has not been found on the Earth’s surface before. Although, I was lucky to meet and work with a man, Boris Nikolaevich Sharupin, who created a material that did not exist on Earth before - boron nitride, the rhombohedral perfect crystals of which he obtained thanks to my research [2,3]. And the highly ordered pyrocrystals of the rhombohedral phase of boron nitride obtained by Sharupin, thanks to their dielectric properties, became the key to understanding the properties of graphite, and layered crystals in general. What is deep inside the Earth, which arose in “Unearthly” Conditions, we, by and large, do not actually know, and what flies to us in the form of asteroids, we also try to analyze in the usual “earthly” concepts. Thus, the diamonds found in the fragments of an asteroid, harder than “earthly”, are trying to be interpreted as nano structured. But this, like “graphene” [4,5], is only a tribute to the NANO-fashion, and NANO cannot change the ultimate characteristics of the crystal of an “earthly” diamond, according to adiabatic decomposition, just as four equivalent electrons of graphite cannot make a purely two-dimensional film of hexagons. In order to affect the basic parameters of the material, it is necessary to use its basic characteristics - chemical bonds of atoms [6-8]. Thus, it is no coincidence that the creation of very hard materials is based on the use of high pressures. But even the use of the explosion method in a high-pressure chamber - pulsed ultra-high pressure of more than 100kBar, does not actually change the bond strength in cubic boron nitride. The error in the recorded reflection peak was determined mainly by the degree of processing of the reflective surface of the crystal, which was approximately equal to diamond in hardness, and exceeded diamond in viscosity (Figure 1).
Figure 1:Anisotropy of lattice oscillators of highly ordered pyrocrystals of the rhombohedral phase and isotropic lattice reflection of cubic boron nitride obtained from the rhombohedral phase by the explosion method. Also shown are the spectra of amorphous boron nitride, in which, as can be seen from the figure, interlayer bonds act predominantly between boron and nitrogen ions.
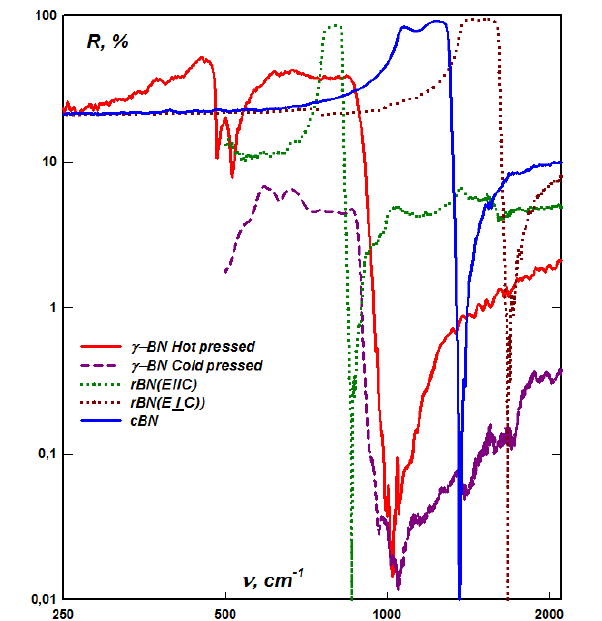
A small inhomogeneity was present in the crystal of cubic boron nitride, which manifests itself in a small asymmetry of the dome of the lattice reflection and in the refractive index in the visible region, which was determined by variations in the edge of the forbidden zone near the value of four and a half electron volts.The lower boundary of the peak of the lattice reflection is determined by the rigidity of the covalent bond of atoms, ions in boron nitride and neutral atoms in graphite. And the upper limit of this peak is determined by the plasma additive due to the dipole formed by boron and nitrogen ions. In graphite, this additive is practically zero, but there is a plasma reflection on free carriers, which screens weak (zero) reflections. So, lattice vibrations in graphite, practically coinciding with the frequency of the lower limit of the lattice reflection of boron nitride, are observed using neutron scattering. As can be seen from the right figure, the anisotropy of the rigidity of the covalent bond of atoms in the crystal lattice of rhombohedral boron nitride, and also in graphite, is approximately one and a half. And taking into account that there are three times fewer interlayer bonds in the lattice (this is also evident from the narrower reflection peak during polarization perpendicular to the hexagonal layers), then the anisotropy - the total rigidity of the bond of the hexagonal layers is weaker inside the layer, only four and a half times. So, in both boron nitride and graphite, as in all layered crystals, the layering is simply due to the fact that planes of weaker bonds produce more defects. Thus, a monoatomic layer of graphite or boron nitride can be torn off with adhesive tape with the same success as a monoatomic layer of silicon from the surface of a crystal. And the chemistry of normal pressures, in fact, has a limit determined, in chemical interactions, by covalent and ionic bonds formed during the interaction of the energy levels of atoms corresponding to the First Ionization Potential. It also actually determines the size of the atoms (Figure 2) and the density of materials, which varies for different elements, but in the condensed state is of approximately the same order for all.
Figure 2(a-e):Regular periodic variations in the size of atoms of chemical elements from the atomic number with the order of magnitude preserved for all atoms.
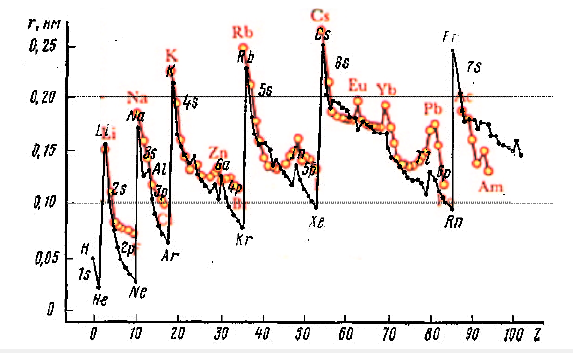
Figure 2 summarizes data from different sources. Quantitatively, they differ slightly, but qualitatively, as can be seen from Figure 2, they agree well. It is generally accepted that Quantum Mechanics completely describes the properties of atoms and that new materials should be designed on its basis. But this is not quite so, or rather, not so at all. Both the atomic radii shown in Figure 2 and the various energy characteristics of the elements have a periodicity corresponding to Mendeleyev’s Law, which simply corresponds to the combinatorics of quantum numbers included in the Schrödinger equation (Figure 2 & 3).
Figure 3:Periodic variations of energy levels of free and condensed chemical elements from the atomic number with the conservation of the order of their values for all atoms.
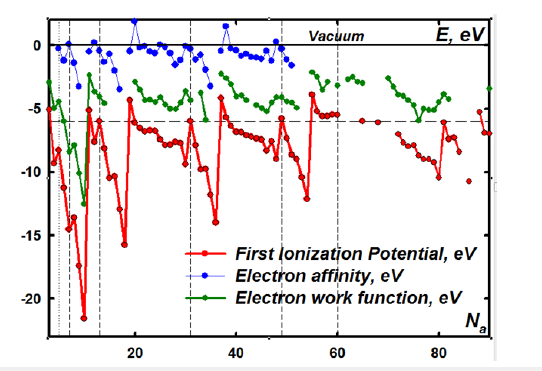
But Planck and Einstein were right to doubt the correctness of this equation [9]. This equation is only a rough adjustment of Reality to incorrectly used mathematical imaginaries [10]. It gives the periodicity of the energy levels of electrons due to the selection (quite reasonable) of quantum numbers, but at the same time it gives a catastrophic discrepancy between the sizes of atoms and the calculated energy levels with the experimental ones with an increase in the atomic number. So modern Quantum Mechanics has described more or less strictly only the hydrogen atom, the model of which is used mainly in solids. And it described the chemical bond in general purely speculatively. So in reasoning it is necessary to start from reliably established empirical experimental patterns, and not from pure fantasies hidden behind scientific sounding “quantum” terminology. Here the use of extremely high pressures allows us to shift slightly into the area of deep levels not taken into account in chemical interactions, which are genetically associated with the Second and subsequent Ionization Potentials [11]. But the Second Ionization Potential for the same atom differs from the First by several times (Figure 4).
Figure 4:Periodic change of the first five ionization potentials from the work [9].
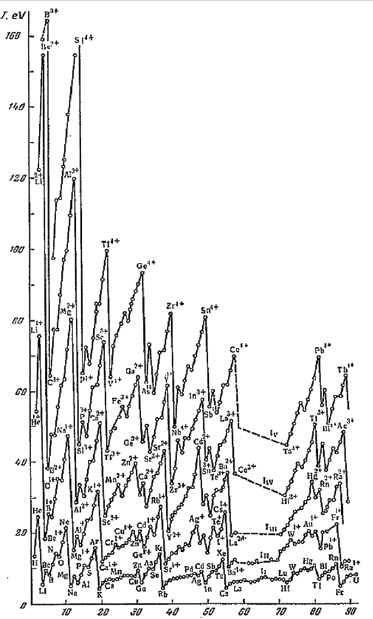
And the excitation of electrons of the inner shells, as can be seen from Figure 4, requires much more energy, by an order of magnitude for light atoms and by orders of magnitude for heavy atoms of the First Ionization Potential.
And the main experimentally established Regularity is that all “normal” materials have basic characteristics of one “earthly” order of magnitude. And this is determined by the fact that the periodic bounce of the energy of the upper electron shell occurs against the background of a monotonically changing average energy level and this bounce lies in a band of one order. So this average energy level for once ionized atoms is fully described by a hydrogen-like quasinuclear model.
And, strictly speaking, the pressures and temperatures technically achievable in the technologies used, as can be seen from experiments with boron nitride, graphite and other refractory materials, are not enough for the chemistry of atomic bonds determined by deep levels to work.
So, to obtain super-dense, super-hard and super-heat-resistant thin coatings, it is necessary to use X-ray irradiation of materials at frequencies resonant with the energy levels of the inner electron shells. In principle, this irradiation technology can produce NANOlayers of a semiconductor with band gaps greater than those of conventional dielectrics.
Of course, it is possible to obtain super-dense materials using irradiation technology, in principle, but only under specific conditions. And first of all, in order to achieve the interaction of deeply ionized atoms, it is necessary that the recombination of electrons from the outer electron shells to the inner ones does not occur. The easiest way to achieve this is in plasma, which is what B.N. Sharupin actually used when growing pyrocrystals of graphite and boron nitride [12]. And it seems that it is necessary to start creating super-dense materials with these ELEMENTARY materials: graphite and boron nitride [13]. Also, in principle, it is possible to start with the technology of high-temperature crystal growth similar to the elementary compound silicon carbide, supplementing it with resonant X-ray irradiation. In this way, it is possible to obtain solid hydrogen and very likely, a new class of superconductors. After all, when forming compounds of atoms due to internal electron shells, the electrons of the outer shells will be generalized with a high probability.
References
- Handbook (1979) USSR academy of sciences, order of lenin institute of general and inorganic chemistry. Physicochemical Properties of Semiconductors. Publishing House “NAUKA”, MOSCOW, Russia, pp. 339.
- Ordin SV, Sharrupin BN, IR polarizer, Patent of SU.
- Ordin SV, Sharrupin BN (1998) Normal lattice oscillations and crystalline structure of non-isotropic modifications of a boron nitride. J Semiconductors (FTP) 32(9): 924-932.
- Ordin SV (2025) Anti-graphene. Open Access Journal of Physics and Science 2(1): 1-6.
- Ordin S (2025) Keynote presentations: ANTIGRAPHEN. Global Conference on Material Science and Nanotechnology, IRIS Scientific Group, Theme: Emerging Trends and Breakthroughs in Material Science and Nanotechnology.
- Ordin S (2018) Electronic levels and crystal structure. Journal of Modern Technology & Engineering 3(2): 125-142.
- Ordin SV (2018) C & BN-Foundation for Atomic-Crystalline Orbitals. Global Journal of Science Frontier Research - Physics & Space Science, GJSFR-A 18(5): 17-47.
- Ordin S (2020) Modern physics. Lambert, pp. 196.
- Ordin S (2024) Foundations of quantization. Jenny Stanford Publishing Pte Ltd, 2024, pp. 494. British Library Cataloguing-in-Publication Data, A catalogue record for this book is available from the British Library.
- Ordin S (2023) Comprehensive analysis of the elementary oscillator. Journal of Electromagnetic Analysis and Applications (JEMAA) 15(5).
- Grigorovich VK (1966) Mendeleev's periodic law and the electronic structure of metals. On the 100th Anniversary of the Discovery of the Periodic Law. Academy of Sciences of the USSR. A.A. Baikov Institute of Metallurgy. Nauka, Moscow, Russia, p. 287.
- Sharupin BN (1976) Structure and properties of boron pyronitride. Shpaka VS, Avarbe RG (Eds.), Chemical gas-phase deposition of refractory inorganic materials. Leningrad, Russia, p. 104.
- Ordin SV (2018) Quasinuclear foundation for the expansion of quantum mechanics. International Journal of Advanced Research in Physical Science (IJARPS) 5(6): 35-45.
© 2025 © Stanislav Ordin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






