- Submissions

Full Text
Research & Development in Material Science
Synthesis and Properties of the Compound TbSbTe3
Zakir Ismailov*, Zivar Hasanova and Rakhila Mirzaeva
Department of General and Inorganic Chemistry, Baku State University, Azerbaijan
*Corresponding author:Zakir Ismailov, Faculty of Chemistry, Department of General and Inorganic Chemistry, Baku State University, Azerbaijan
Submission: August 29, 2025;Published: September 16, 2025

ISSN: 2576-8840 Volume 22 Issue 3
Abstract
Chemical interaction in the Sb2Te3 - Tb2Te3 system was studied using physicochemical analysis methods (differential thermal analysis, X-ray phase analysis, microstructural analysis, microhardness measurement and density determination). Based on the analysis results, a phase diagram of the Sb2Te3 - Tb2Te3 system was constructed. It was found that the Sb2Te3 - Tb2Te3 section is a quasi-binary section of the Tb-Sb-Te ternary system and belongs to the eutectic type. It is evident from the phase diagrams that in the Sb2Te3 - Tb2Te3 section, a new ternary incongruently melting compound of the TbSbTe3 composition is formed by a peritectic reaction at a temperature of 980K with a component ratio of 1:1. According to the results of X-ray phase analysis, it was established that the TbSbTe3 compound crystallizes in orthorhombic syngony with the lattice parameters: a =2.35, b=12.76, c=4.86 Å. The solubility of Tb2Te3 in Sb2Te3 is 3.0 mol.% at 300K, respectively. The electrophysical properties of the TbSbTe3 compound have been studied. It has been shown that this compound is an «n»-type semiconductor, with a band gap of ~0.21eV.
Keywords:System; Analysis; Crystallization; Phase; Diagram; Temperature
Introduction
Modern scientific and technical progress is inextricably linked with the development of semiconductor technology [1-3]. The rapid development of the latter was the main stimulus for the search for complex semiconductor materials [4-6]. However, the growing need for semiconductor technology in materials is not yet fully satisfied due to the lack of materials with different combinations of optical, magnetic and electrophysical properties.
Chalcogenides of antimony of the composition Sb2X3(X-Se, Te) and solid solutions based on them are used as a thermoelectric material in the manufacture of n-branches of thermoelectric devices [7,8]. Chalcogenides of rare earth elements and alloys based on them are promising compounds for the development of thermal materials [9-11]. Therefore, obtaining new materials based on them is an urgent task [12-15].
Experimental Part
The TbSbTe3 compound is obtained by synthesizing the Sb2Te3 - Tb2Te3 compounds. Synthesis of the Sb2Te3 - Tb2Te3- system. Terbium TbM-1 purity 99.9%, antibium grade (B-4) and Te, purity 99.999% were used for the synthesis of the samples.
The synthesis mode was selected based on the physicochemical properties of the elemental components and binary compounds. The ligatures were first crushed and finely ground, weighed on an analytical balance with an accuracy of one part per thousand, and filled into quartz ampoules.
The ampoules were evacuated to a pressure of 10⁻³mm.cv.st using a vacuum apparatus and their mouths were sealed by soldering using an oxygen gas flame. This was achieved by direct melting of the components in evacuated quartz ampoules at 1600K for 6 hours, followed by slow cooling when the furnace was turned off.
The ligatures were first crushed and ground into fine powder, weighed on an analytical balance to the nearest thousandth, and filled into quartz ampoules. The ampoules were evacuated to a pressure of 10⁻³ mm.cv.st using a vacuum apparatus, and the mouths were sealed by soldering using an oxygen gas flame.
Samples with a content of 60 mol% Tb2Te3 and higher were obtained as sinters. They were crushed again and turned into tablets. Alloys with a content of less than 60 mol% are compact, dark gray in color with a metallic luster. After synthesis, in order to achieve homogeneity of the alloy, 250g were additionally annealed at a temperature 50-100K below the solidus. The obtained samples were subjected to detailed physicochemical studies.
To achieve homogeneity of the alloy after synthesis, it was additionally annealed at temperatures 50-100K below the solidus for 250g. The obtained samples were subjected to detailed physicochemical study. The heating and cooling curves of the alloys were recorded on a Termoksan, X-ray diffraction was performed on an X-ray diffractometer.
Differential thermal analysis alloys of the system were carried out on a TERMOSKAN-2 device with an accuracy of 3-5 °C, a chromel-alumel thermocouple, and calcined Al2O3 served as the standard. Heating rate of 9 degrees/min. High-temperature differential thermal analysis was carried out on a device (HTDA) - 8m2 in an inert atmosphere using a B-B/Re thermal suspension, degreasing speed of 5rev/min.
X-ray phase analysis was performed on an X-ray instrument of the D2 PHASER model through the use of CuKα radiation with a Ni filter. The micro-structural analysis of alloys was carried out using an MIM-8 microscope. In the study of alloy microstructure, an etchant of composition 1 N HNO3 + HF = 2:1 was used, the etching time was 20s. The microhardness of the phases was measured on a PMT-3 instrument with an accuracy of 5%, and the density of the samples was determined by the pycnometric method.
Results and Discussion
Based on the results obtained by the above methods, a phase diagram of the Sb2Te3 - Tb2Te3 system was constructed (Figure 1).
Figure 1:State diagram of the Sb2Te3- Tb2Te3 system.
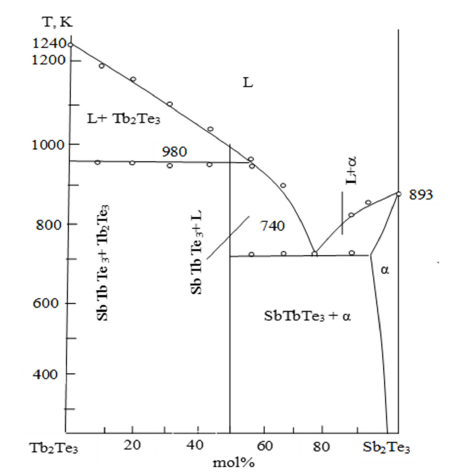
As can be seen from the figure, the system is quasi-binary and
eutectic. It is clear from the phase diagram of the Sb2Te3- Tb2Te3
system that the peritectic formation of the compound containing
TbSbTe3 in a 1:1 ratio occurs at a temperature of 980K.
L + Tb2Te3 ↔ TbSbTe3
A solubility range of up to 3mol.% has been found at 300K. TbSbTe3 forms a eutectic with Sb2Te3 at 82mol.%. The solubility of Tb2Te3 in Sb2Te3 at 300K is 3mol%.
X-ray diffraction patterns of TbSbTe3 powders have been indexed to show that the compound crystallizes in an orthorhombic syngony with a Sb2S3-type structure. The lattice parameters are a=12.35, b=12.76, c=4.86Å.
This process is important for studying the interactions of elements in materials and for more accurately determining their nature (Table 1).
Table 1:Expression of the compositions of Sb2Te3- Tb2Te3 system alloys.
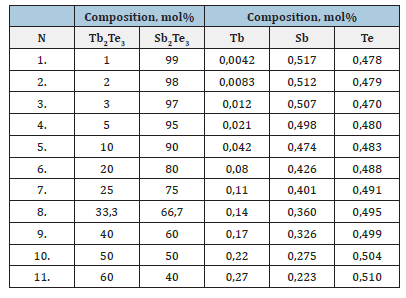
The effect of various reagents on the alloys was studied. It was found that they are not affected by “organic solvents” - benzene, toluene, as well as water and air, but are decomposed by the action of mineral acids and alkalis. To achieve homogeneity of the samples, they were annealed at a temperature of 500-600K for 200 hours.
The thermally treated samples were cooled to room temperature and then subjected to complex physicochemical studies. This process is important for studying the stability of the alloy and its behavior in various conditions, as well as for more accurately determining the structure and properties of the substance.
According to the DTA (differential thermal analysis) results of the alloys, all effects observed in the heating curves are endothermic and reversible.
The results of the X-ray diffraction analysis showed that the X-ray reflections of the alloy obtained in a 1:1 ratio of the components differ sharply from those of the original components. This indicates the formation of a new phase in the system in a 1:1 ratio. This finding confirms the DTA results. According to the MQA results, all samples, except for the samples with a content of 0-5 and 50 mol%, are two-phase. This also supports the DTA and X-ray diffraction results. The study of such multiphase systems provides deeper information about the structural and physical properties of the alloy.
Electrophysical properties of the compound TbSbTe3
Since Sb2Te3 is a semiconductor material with a defect structure, it is necessary to study the temperature dependence of the electrical conductivity and thermoelectric coefficient of terbium and its ternary telluride alloys in the solid solution region based on it.
The temperature dependence of the specific electrical conductivity (σ) of the TbSbTe₃ compound is semiconducting up to about 450K above room temperature (Figure 2). The ternary compound corresponds to impurity conductivity in this range, while above 500K it exhibits intrinsic conductivity properties.
Figure 2:Temperature dependence of the electrical conductivity (σ) of the TbSbTe3 compound.
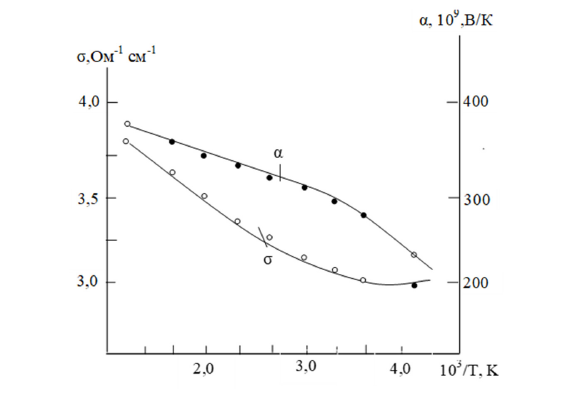
The figure shows that α increases with increasing temperature. Later, its saturation is observed, and then a decrease occurs. While σ first decreases, then increases.
This unusual feature suggests that the thermoelectric power variation is related to semiconductors with complex energy band structures. Based on this, it can be assumed that the newly formed TbSbTe3 compound also has a complex energy band structure and appears to be similar to the energy level structure of bismuth sesquitelluride [16-18].
The calculated energy band gap (ΔE) for TbSbTe3 is approximately 0.21eV. At the same time, the variation of the thermoelectric coefficient (α) of the TbSbTe3 compound with temperature is also demonstrated. Based on the thermoelectric power and Hall coefficients, the TbSbTe3 compound was determined to be an «n»-type semiconductor.
Conclusion
1. The interaction in the Tb2Te3-Sb2Te3 system was investigated
and a phase diagram of the system was constructed using
differential thermal, X-ray phase, microstructural analyses,
and microhardness measurement methods.
2. It has been found that the Tb2Te3-Sb2Te3 system is quasi-binary.
As a result of a peritectic reaction in the system, the TbSbTe3
compound is formed.
3. The electrophysical properties of the TbSbTe3 compound have
been studied. It has been shown that this compound is an «n»-
type semiconductor, with a band gap of ~0.21eV.
References
- Yarembash EI, Eliseev AA (1975) Chalcogenides of rare earth elements. Moscow, Russia, p. 275.
- Lyakishev RPM (1997) Phase diagrams of binary metallic systems. Mechanical Engineering, p. 1023.
- Ghosh S, Moreira MVB, Fantini C, González JC (2020) Growth and optical properties of nanocrystalline Sb2Se3thin-films for the application in solar-cells. Solar Energy 211: 613-621.
- Sankapal BR, Lokhande CD (2001) Studies on photoelectrochemical (PEC) cell formed with SILAR deposited Bi2Se3-Sb2Se3 multilayer thin films. Sol Energy Mater Sol Cell 69: 43-52.
- Li W, Deng L, Wang X, Cao J, Xie Y, et al. (2021) Close-spaced thermally evaporated 3D Sb2Se3film for high-rate and high-capacity lithium-ion storage. Nanoscale 13: 9834-9842.
- Xue MZ, Fu ZW (2008) Pulsed laser deposited Sb2Se3 anode for lithium-ion batteries. J Alloys Compd 458: 351-356.
- Zhang H, Liu CX, Qi XL, Dai X, Fang Z, et al. (2009) Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat Phys 5: 438.
- Fedorov VI (1969) On the thermal conductivity of liquid antimony chalcogenides. High Temperature Thermal Physics 7(2): 269-275.
- Zhen Li, Chen Si, Jian Zhou, Huibin Xu,Zhimei S (2016) Yttrium-doped Sb2Te3: A promising material for phase-change memory. ACS Appl Mater Interfaces 8(39): 26126-26134.
- Sadygov FM (1993) Physico-chemical basis for the production of chalcostibines and chalcobismuthites of rare earth elements and materials based on them, doctoral dissertation. Baku, p. 48.
- Shenyamova LE, Karpiyskiy OG, Konstantinov PP, Svechnikova TE, et al. (2006) Layered chalcogenides in quasi-binary systems AIVBVI - AV2BVI (AIV - Ge, Sn, Pb; BV - Bi, Sb, BVI - Te,Se) promising thermoelectric materials for thermos generators. Advanced Materials 3: 5-17.
- Suh J, Yu KM, Fu D, Liu X, Yang F, et al. (2015) Simultaneous enhancement of electrical conductivity and thermopower of Bi2Te3 by multifunctionality of native defects. Adv Mater 27: 3681-3686.
- Ghosh G (1994) The Sb-Te (Antony-Tellurium) system. Journal of Phaze Equilibria 15: 349-360.
- Novitsky AP, Voronin AI, Usenko AA, Faershteyn KL, Khovaylo VV (1915) Materials based on bismuth and antimony chalcogenides obtained by spinning. National University of Science and Technology "MISiS", Conference Proceedings pp. 85-90
- Lavrentiev MG, Osvenskiy VB, Pivovarov GI, Sorokin AI, Karataev VV, et al. (2014) Mechanical properties of solid solutions of bismuth and antimony chalcogenides obtained by directional crystallization and powder metallurgy methods. Collection of proceedings of the conference Thermoelectric and their applications, St. Petersburg, pp. 307-312
- Mashkarev TV, Paderko YuBZh (1965) Neorgan. Materialy 1(10): 179.
- Nedoma I (1975) Decoding X-ray diffraction patterns of powders. Rastorguev LN (Ed.), Metallurgy, p. 423.
- Collection (1960) Semiconductor substances. Questions of chemical bonding. Zhuze VP (Ed.), p. 268.
© 2025 © Zakir Ismailov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






