- Submissions

Full Text
Research & Development in Material Science
Synthesis and Characterization of Coconut Shell-Based Nanobiochar Produced by Controlled Pyrolysis for Dye Adsorption from Wastewater
Deshraj Singh Thakur
Santosh Narayan Chadar*
Govt Auto Girls PG, Excellence College Sagar, India
*Corresponding author:Santosh Narayan Chadar, Govt Auto Girls PG, Excellence College Sagar, India
Submission: August 28, 2025;Published: September 09, 2025

ISSN: 2576-8840 Volume 22 Issue 2
Abstract
This study outlines the synthesis, physicochemical characterization, and pollutant removal capabilities of nanobiochar derived from coconut shell biomass. The biochar was produced through controlled pyrolysis at 800 °C for 3 hours and subsequently milled to achieve nanoscale dimensions. Dynamic light scattering analysis indicated an average particle size of 618.1nm with a polydispersity index, suggesting a moderately uniform nanoscale distribution. Structural analyses, complemented by BET surface area measurements, confirmed prominent aromatic features, several functional groups that include oxygen, and a large specific surface area of 422.7m²/g. The nanobiochar demonstrated a zeta potential of -83.38mV, indicating excellent colloidal stability. Batch adsorption experiments revealed high sorption capacities for methylene blue and Pb, with removal efficiencies exceeding 95% under optimal conditions. The pseudo-second-order Langmuir isotherm kinetics models offered the best fit for equilibrium and kinetic studies, respectively, suggesting a primary chemisorption process. These findings highlight coconut shell-derived nanobiochar as an economical and environmentally pleasant adsorbent for wastewater treatment and remediation.
Keywords:Coconut shell biomass; Nanobiochar; Pyrolysis; Surface area; Adsorption; Methylene blue; Water purification; Environmental remediation
Introduction
The escalating global concern over water contamination necessitates the development of innovative and sustainable water purification solutions, driving research into advanced materials capable of efficiently removing pollutants from aquatic environments [1]. Nanobiochar, a nanoscale manifestation of biochar, has garnered attention as a material with significant potential, attributed to its substantial surface area, controllable porosity, and capacity for surface alteration, all of which bolster its adsorptive properties [2]. Biochar, generally, is a material rich in carbon produced through pyrolysis of biomass in low-oxygen conditions, usually between 300 °C and 1000 °C [3]. Its natural properties, such as high surface area and porosity, make it a valuable adsorbent for various contaminants, including heavy metals, organic dyes, and pesticides. The source material and temperature of pyrolysis have a significant impact on how well biochar works as an adsorbent [4]. Producing biochar from waste materials offers an economical method and promotes environmental sustainability. Coconut shells, an abundant and often underused agricultural waste, serve as a sustainable and cost-effective source for nanobiochar production, potentially turning waste into a valuable tool for environmental cleanup. Developing efficient, affordable, and eco-friendly tertiary treatment methods has become increasingly urgent due to growing environmental concerns. Traditional treatment processes often struggle to sufficiently reduce or eliminate pollutants,but biochar has applications in areas like wastewater treatment, greenhouse gas reduction, and pollution cleanup [5]. Nanobiochar’s unique physicochemical traits provide improved performance over traditional biochar in water treatment [6]. Adding nanomaterials onto biochar’s surface can enhance its properties by changing functional groups, porosity, and active sites, boosting its ability to trap heavy metals [7]. The larger surface area and higher reactivity of nanobiochar allow for better interactions with pollutants, resulting in faster adsorption rates and greater capacity [8]. The mechanisms behind pollutant removal by biochar are complex and include pore filling, electrostatic attraction, ion exchange, precipitation, and surface adsorption [9]. The adsorption of organic pollutants and heavy metals is greatly enhanced when biochar is modified with nanoparticles. Physicochemical parameters of the nanobiochar, including surface area, pore size distribution, and surface charge, as well as the characteristics of the contaminants, determine the unique adsorption mechanisms. Temperature, residence time, and heating rate are all important pyrolysis parameters that must be optimized in order to customize nanobiochar’s characteristics for certain water treatment uses. Screening, grinding, pH balancing, and activation by chemical or physical treatments to enhance surface area and encourage ion exchange are examples of additional biochar processing steps after pyrolysis [10]. The synthesis of Nanobiochar from coconut shells typically involves a controlled pyrolysis process, where the biomass is heated in an inert atmosphere to induce thermal decomposition. The pyrolysis temperature plays a critical role in determining the properties of the resulting nanobiochar, with higher temperatures generally increasing surface area and carbon content but potentially reducing the yield. The particle size of biochar varies depending on the pyrolysis technique used, ranging from micrometers to centimeters [11]. The physicochemical characteristics of nanobiochar, such as its surface area, pore size distribution, elemental content, and surface functional groups, are evaluated using a variety of analytical techniques. Methods such as transmission electron microscopy, atomic force microscopy, and scanning electron microscopy are employed to visualize the size and shape of the nanobiochar particles. The degree of graphitization and crystalline structure of the carbon material can be inferred by X-ray diffraction and Raman spectroscopy. In order to detect and measure surface functional groups-which are essential for pollutant adsorption-Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy are used. Chemical alteration increases surface area and functional groups, which generally results in a better adsorption capacity than unmodified biochar [12]. This study aims to synthesize nanobiochar from coconut shells through a controlled pyrolysis process and thoroughly characterize its physicochemical properties. Additionally, the research explores the application of this nanobiochar in removing specific water pollutants, assessing its adsorption performance and the mechanisms involved. Coconut fiber biomass has shown a surface area of 23.9145m2/g [13]. The novelty of this research lies in optimizing the pyrolysis process for coconut shell nanobiochar production and conducting a detailed investigation of its efficacy in removing multiple types of water pollutants, providing insights into its potential as a versatile and sustainable water treatment material. The characteristics of biochar are strongly influenced by variables such as moisture, ash, volatile matter, and fixed carbon. Nanobiochar was produced using ball milling under optimal parameters, then conditioned for 24 hours at -80 degrees Celsius, resulting in an average particle size of about 60nm [14]. Understanding biochar’s properties requires considering its elemental composition-carbon, hydrogen, oxygen, and nitrogenand surface features, pore volume, pore size, and surface functional groups [15,16]. The ultimate goal of this research is to aid in the creation of economical, environmentally friendly water treatment systems. increasing environmental sustainability and the worldwide problem of water contamination [17]. The characteristics of biochar can be changed using different chemical or physical agents to suit different uses, according to research efforts [18]. This study adds to the expanding collection of research and real-world applications of biochar by meticulously analyzing the synthesis, characterization, and use of coconut shell-based nanobiochar in water treatment. The rise in pyrolysis temperature frequently leads to a higher carbonized fraction and larger surface area of biochar, which in turn leads to a high sorption capability for pollutants [19]. The increasing interest in biochar for environmental applications stems from its capacity to act as an effective and sustainable carbon sequestration agent, simultaneously improving soil quality and promoting plant growth [20]. A sustainable approach to resource recovery and waste management is provided by biochar, a carbonrich substance made by pyrolyzing biomass [21,22]. The effectiveness of biochar as a soil amendment and carbon sequestration agent hinges on its physical and chemical properties, which are intricately linked to the type of biomass used and the pyrolysis conditions employed [23]. Biochar’s high surface area, cation exchange capacity, and presence of functional groups make it a promising candidate for enhancing methane production, reducing lag phases, and adsorbing inhibitors in anaerobic digestion processes [24,25]. Biochar can be produced from a variety of biomass sources, including agricultural residues, wood waste, and municipal solid waste, making it versatile and adaptable material for different regions and applications [26,27]. The characteristics of biochar are heavily influenced by a number of variables, which include moisture, ash, volatile matter, and fixed carbon [28]. Understanding of biochar’s characteristics requires consideration of ultimate, hydrogen, oxygen, and nitrogen composition. and surface characteristics, pore volume, pore diameter, and surface functional group. Biochar’s capacity to adsorb pollutants from both soil and water environments has garnered significant attention, providing a promising avenue for environmental remediation. The use of biochar in anaerobic digestion has proven effective in promoting syntrophic relationships between microorganisms, leading to enhanced organic matter degradation and biogas production. Biochar produced at higher temperatures tends to have a more aromatic structure, enhanced stability and carbonization, as evidenced by a greater specific surface area and lower H/C and O/C ratios. The application of biochar in anaerobic digestion not only enhances biogas production but also facilitates nutrient recovery from digestate, closing the loop in waste management [29].
Materials and Methods
Raw material collection and pre-treatment
Coconut shells, a waste product from homes in Kerala, India, were thoroughly cleaned. First, they were washed with tap and distilled water to remove dirt and organic matter. Then, the shells were soaked in acetone for two hours to get rid of leftover oils and volatile substances. After this, the shells were dried in a hot air oven at 105 °C for 24 hours to remove all moisture. The dried shells were then left in the sun for 72 hours to stabilize. Finally, the stabilized shells were ground and sieved to create particles less than 2mm in size for further use. All chemicals used, such as methylene blue dye, hydrochloric acid, and sodium hydroxide, were analytical grade and bought from Merck. Deionized water was used as the solvent for all experiments [30].
Pyrolysis for nanobiochar production
Biochar was produced from cleaned and pretreated coconut shell powder through slow pyrolysis in a sealed SS310 tubular reactor. A quantity of roughly 25g of dried feedstock was heated at a rate of 10 °C min⁻¹ to a peak temperature of 800 °C, kept at this temperature for three hours, and then permitted to cool naturally within the reactor. The resultant biochar, characterized by its black color and brittleness, was collected and stored in an airtight container to prevent any degradation or contamination. For the synthesis of nanobiochar, the bulk biochar underwent a topdown mechanical grinding process. Subsequently, it was subjected to probe ultrasonication in an aqueous environment, followed by low-speed centrifugation, which facilitated the effective isolation of nanoscale components [31].
Characterization of nanobiochar
The fabricated material was thoroughly analyzed using a comprehensive array of methods, encompassing Scanning Thermogravimetric analysis, CHNS/O elemental analysis, X-ray Diffraction, Raman Spectroscopy, Fourier-Transform Infrared Spectroscopy, X-ray Photoelectron Spectroscopy, Electron Microscopy, Transmission Electron Microscopy, Brunauer-Emmett- Teller surface area analysis, and Dynamic Light Scattering with zeta potential measurements. The results showed that partial graphitization and the development of a hierarchical porous structure were caused by raising the pyrolysis temperature. Nanoscale pieces with enhanced surface accessibility and a higher concentration of edge sites were produced by subsequent top-down mechanical processing, which eventually improved the electrochemical performance and adsorption capabilities [32,33]. The physicochemical attributes of nanobiochar derived from coconut shells were thoroughly investigated to ascertain its potential for environmental applications. Fourier Transform Infrared spectroscopy indicated the presence of oxygenated functional groups crucial for adsorption processes. X-ray Diffraction patterns exhibited broad peaks at approximately 23° and 43° (2θ), suggesting an amorphous carbon structure with disordered graphitic regions. Scanning Electron Microscopy images revealed a highly porous and irregular morphology characterized by numerous cavities, correlating with an increased surface area and a high density of adsorption sites. Brunauer-Emmett-Teller analysis confirmed the presence of mesoporosity, a substantial surface area, significant pore volume, and an appropriate pore diameter, all of which are conducive to both adsorption and catalytic activities. Moreover, zeta potential measurements indicated a negative surface charge, attributed to the presence of carboxylate and hydroxyl groups, which promotes colloidal stability and facilitates effective electrostatic interactions with cationic contaminants. In aggregate, these findings establish that coconut shell-derived nanobiochar exhibits favorable physicochemical properties, including a large surface area, hierarchical porosity, and abundant surface functionalities, thereby positioning it as a potent material for the remediation of heavy metals, dyes, and other environmental pollutants [34-36].
Figure 1:Adsorption efficiency of coconut shell-derived nanobiochar (CS-NBC) evaluated using methylene blue (MB) as a model organic contaminant.
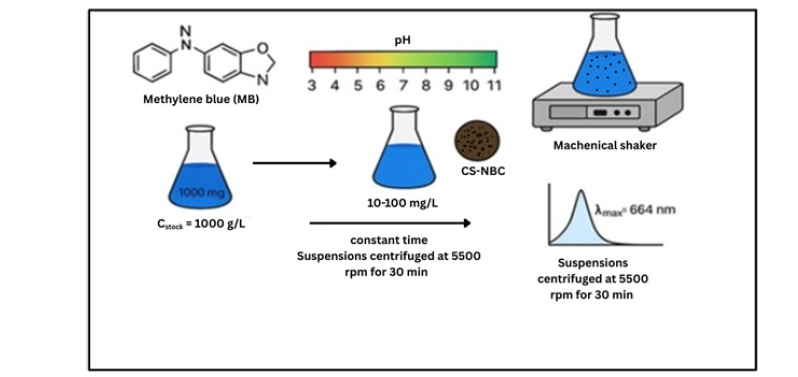
Batch adsorption studies were used to evaluate the impact of pH, contact time, and initial dye concentration on the adsorption of methylene blue, a typical organic pollutant, using nanobiochar made from coconut shells. Working concentrations ranging from 10 to 100mg L⁻¹ were created by diluting a stock solution of methylene blue made with deionized water. A standard adsorption experiment involved adding 0.1g of CS-NBC to 100mL of the methylene blue solution in a 250mL Erlenmeyer flask. 0.1M HCl or 0.1M NaOH was used to change the pH of the solution from 3 to 11, depending on the particular experimental settings. After that, the flasks were shaken at 150rpm for varied periods of time at room temperature in order to study the adsorption kinetics. To separate the adsorbent, the mixtures were centrifuged for 10 minutes at 5000rpm following the prearranged contact time. The concentration of methylene blue that remained in the supernatant was then measured using UV-Vis spectrophotometry [37]; (Figure 1).
Data analysis
The efficacy of nanobiochar derived from coconut shells in adsorbing organic contaminants was assessed using methylene blue as a representative pollutant. Adsorption experiments were performed in batches, systematically altering initial dye concentrations, contact duration, and the amount of adsorbent used. Following the attainment of equilibrium, the remaining methylene blue concentration was quantified at 664 nm via UVVis spectrophotometry. The calculation of adsorption capacity and removal efficiency was performed utilizing the subsequent formulas:
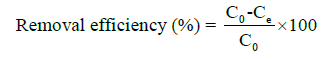
C0 = Initial concentration of adsorbate (mg/L)
Ce = Equilibrium concentration of adsorbate (mg/L)
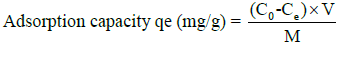
V = Volume of solution (L)
M = Mass of adsorbent (g)
The adsorption mechanism was investigated by fitting experimental data to well-known isotherm models, including the Freundlich and Langmuir models. Additionally, adsorption kinetics were studied using pseudo-first order and pseudo-second-order kinetic models. Each model’s appropriateness was determined by evaluating its correlation coefficient and alignment with the experimental results [38].
Results and Discussion
Yield of coconut shell-based nanobiochar
The yield of nanobiochar, quantified as a percentage, is determined by the proportion of nanobiochar mass recovered post-pyrolysis relative to the initial biomass mass. This value serves as an indicator of the effectiveness of biomass transformation into a stable carbonaceous substance and is contingent upon pyrolysis parameters, including temperature, heating rate, and duration. Typically, elevated pyrolysis temperatures tend to reduce yields but concurrently enhance the carbon content and surface characteristics of the resultant nanobiochar [39].
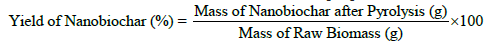
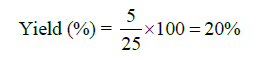
Elemental composition (EDX Analysis)
Figure 2:SEM-EDS elemental mapping of coconut shell-derived nanobiochar (CS-NBC).
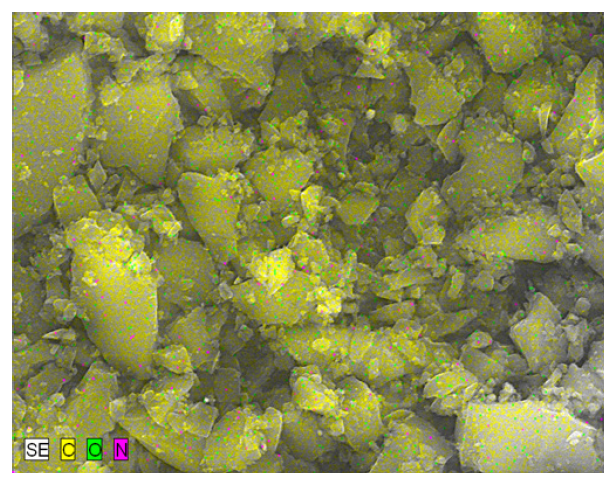
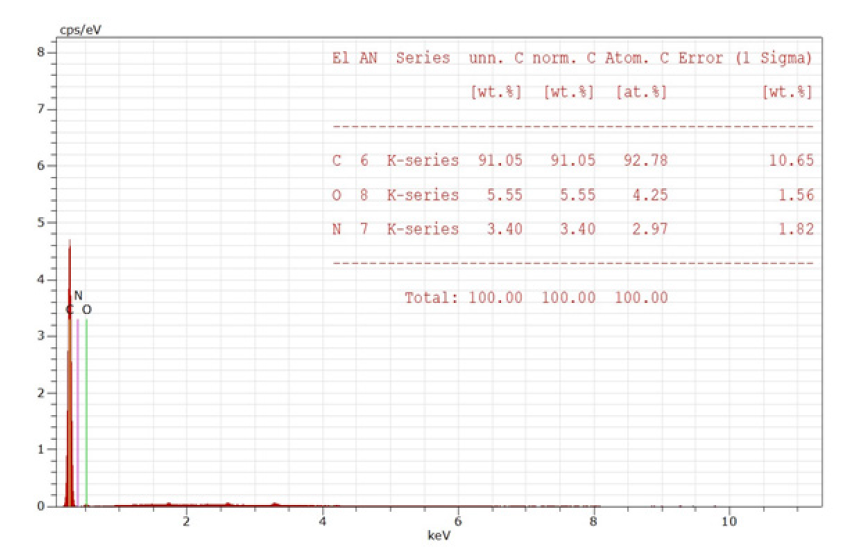
Dispersive X-ray energy. Using spectroscopy, the elemental composition of the coconut shell-derived nanobiochar was ascertained. With corresponding masses of about 78.4% and 19.6%, carbon and oxygen were the most prevalent elements, according to the EDX spectra. Silicon, calcium, and potassium were also found in trace amounts. The high-temperature pyrolysis technique, which promotes carbonization and strengthens aromatic structure, uses heat to break down cellulose, hemicellulose, and lignin, which accounts for the significant carbon content [40]. This EDS spectrum indicates the sample is overwhelmingly carbonbased: about 91wt% carbon (≈92.8at.%), with minor oxygen (~5.6wt%, 4.3at.%) and nitrogen (~3.4wt%, 3.0at.%). There are no heavier elements visible across the rest of the energy range, and the indicated K-series peaks at low keV correspond to C, O, and N. This composition points to a carbonaceous substance (carbon film, polymer, etc.) with trace amounts of nitrogen and oxygen, most likely from surface contamination, oxidation, or functional groups. The numbers in the rightmost column should be considered as approximate rather than accurate because the 1-sigma errors are comparatively bigger for carbon because of low-energy X-rays and matrix effects (Figure 2 & 3).
Figure 3:EDS spectrum of coconut shell-derived nanobiochar (CS-NBC) showing the elemental composition of C, O, and N.
FTIR spectroscopy
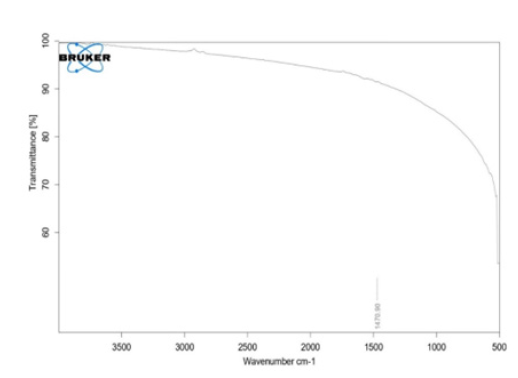
The Fourier Transform. The surface functional groups of the nanobiochar were characterized using infrared spectroscopy. The large absorption band at about 3430cm-1, which is suggestive of O-H stretching vibrations, verified the presence of hydroxyl groups. The peaks located at around 2920 and 2850cm-1 were found to be aliphatic C-H stretching vibrations. Additionally, a significant degree of carbonization was suggested by a peak at about 1620cm- 1 that was attributed to C=C stretching inside aromatic rings [41]. The identification of spectral bands near 1100cm-1 suggests the presence of C-O bond stretching vibrations, characteristic of alcohols or ethers. These oxygen-containing functional groups are noteworthy for their ability to enhance surface polarity, which in turn promotes the adsorption of pollutants. The intensity of the studied phenomena was diminished as a result of hightemperature pyrolysis. This observation aligns with findings of improved carbonization and graphitic restructuring [42]. This FTIR transmission spectrum map (transmittance vs. wavenumber) reveals a noticeable fall-off below ~700cm-1 (with a sharp edge near 500cm-1) and a very high overall transmission (about 95-100%) in the mid-IR region, with a gentle, steady reduction toward lower wavenumbers. The low wavenumber drop most likely reflects either the intrinsic absorption/phonon features of the sample or, more frequently in such broadband optics, the transmission cutoff of the window/substrate or the instrument detector response. The nearly flat, high-transmission baseline suggests a mostly transparent sample or empty beam/reference measurement. It looks more like a reference or cursor line than a high absorption peak at the thin vertical marker at about 1470cm-1. The figure indicates that, aside from the instrument/window-limited region at the far-IR end, there is little sample absorption in the observed range overall (Figure 4).
Figure 4:Functional group identification of coconut shell nanobiochar by FTIR.
X-ray Diffraction (XRD)
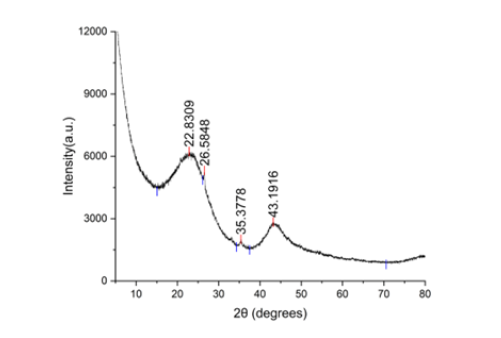
The X-ray diffraction analysis of the nanobiochar reveals a composite structure, featuring both amorphous and partially crystalline components. A wide diffraction band near 2θ≈22.3° is indicative of the cellulose plane, suggesting a largely amorphous carbon matrix typical of biochar derived from biomass. Additionally, diffraction peaks identified at 2θ≈26.58°, 35.38°, and 43.19° align with the characteristic reflections of graphitic carbon planes, signifying the presence of limited ordered graphitic domains formed during the pyrolysis process. The calculated crystallinity index, based on the ratio of crystalline to total peak intensity, confirms a low degree of structural order. This amalgamation of highly disordered carbon interspersed with nascent graphitic regions optimizes adsorption capabilities through an abundance of surface functional groups, while concurrently bolstering electrical conductivity and structural robustness. Consequently, the nanobiochar is well-suited for uses where heavy metals and organic pollutants need to be adsorbed, alongside potential utility in energy storage and catalytic transformations [43]. The XRD pattern further presented a broad peak centered around 2θ≈24.6°, characteristic of amorphous carbon, and a less pronounced shoulder near 2θ≈43.7°, attributed to graphitic structures with a degree of disorder [44]. Plot labels indicate that this X-ray powder diffraction pattern has multiple large peaks at 2θ=22.28°, 26.58°, 35.38°, and 43.19°. When the peak positions are converted to interplanar spacings (using Cu Kα, λ≈1.5406Å), the resulting d≈3.99Å, 3.35Å, 2.53Å, and 2.09Å, respectively. The 26.6°/3.35Å feature is frequently linked to the (002) spacing of graphitic/stacked carbon, while the broadness of all peaks (as well as the background that falls smoothly toward high angle) suggests low crystallinity, very small crystallite size and/or microstrain, and possibly an amorphous component. To index phases or reliably identify crystalline species, the relative intensities and precise peak positions are required. If you supply instrumental parameters, a Scherrer analysis could estimate the size of the crystallites (Figure 5).
Figure 5:XRD pattern of the coconut shell-based nanobiochar synthesized sample showing characteristic diffraction peaks.
Scanning Electron Microscopy (SEM)
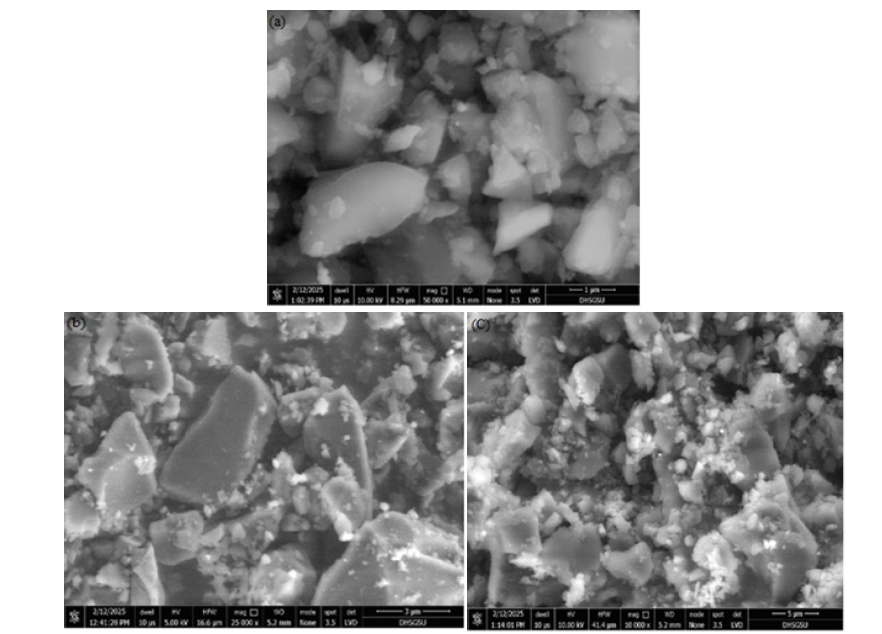
Scanning electron microscopy indicated a surface texture that was both rough and porous, featuring irregular voids and interconnected formations. These characteristics are indicative of volatile material release and structural breakdown during the pyrolysis process. The high-temperature treatment also fostered substantial pore expansion and surface fracturing, which are advantageous for adsorption processes. Additionally, the observation of nanoporous elements points to potential for extensive surface area, a finding corroborated by Brunauer- Emmett-Teller analysis. Comparable morphological attributes have been documented in biochar derived from biomass subjected to similar high-temperature pyrolysis conditions [45]. Scanning electron microscopy images delineate the surface topography of coconut shell-derived nanobiochar across multiple magnifications. The micrographs exhibit a heterogeneous architecture composed of irregularly shaped, angular particles with uneven surfaces and a spectrum of sizes, from nanometer-scale crystallites to larger micrometer-scale fragments. The superior micrograph emphasizes the presence of fine nanoparticles adhered to larger agglomerations, indicative of a substantial surface area. The lower left micrograph reveals lamellar particles characterized by acute edges and fissures, whereas the lower right micrograph displays a more porous and granular texture with clusters and voids. Cumulatively, these morphological attributes affirm the formation of rugged, porous surfaces conducive to elevated adsorption capabilities, thereby positioning the nanobiochar as an efficacious agent for the remediation of methylene blue dye (Figure 6).
Figure 6:SEM images of coconut shell-derived nanobiochar (CS-NBC) at different scales: (a) 1μm, (b) 3μm, and (c) 5μm.
Brunauer-Emmett-Teller (BET) surface area
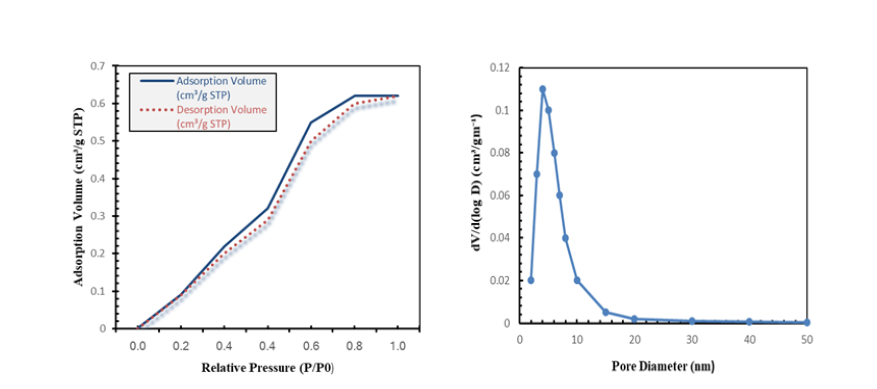
The BET study of CS-NBC revealed a mesoporous structure with an average pore diameter of 5.4nm, a surface area of 422.7m²/g, and a total pore volume of 0.62cm³/g. Mesopores were confirmed by the nitrogen adsorption-desorption isotherm, which showed a hysteresis loop and a Type IV categorization [46]. Because there are more accessible active sites due to the material’s mesoporous structure and increased surface area, both organic and inorganic pollutants can be adsorbed more easily. The process of devolatilization and the reorganization of the carbon matrix at high pyrolysis temperatures are responsible for this improvement in characteristics [47]; (Figure 7).
Figure 7:N₂ adsorption-desorption isotherm (a) and BJH pore size distribution (b) of CS-NBC, confirming mesoporosity (~5.4nm).
Zeta potential (mobility and particle size analysis)
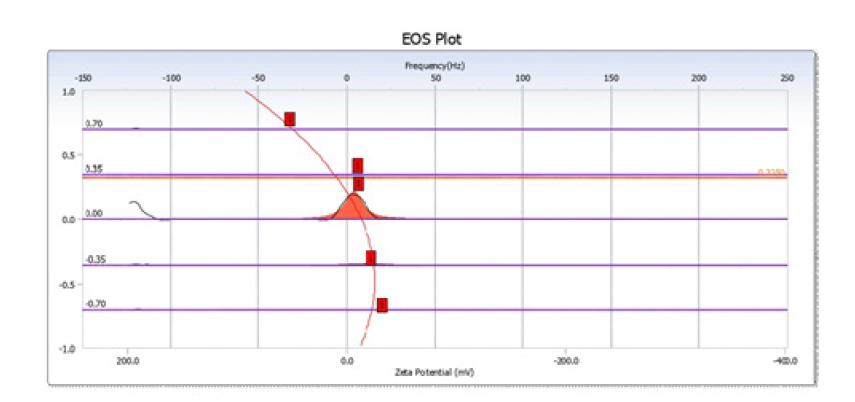
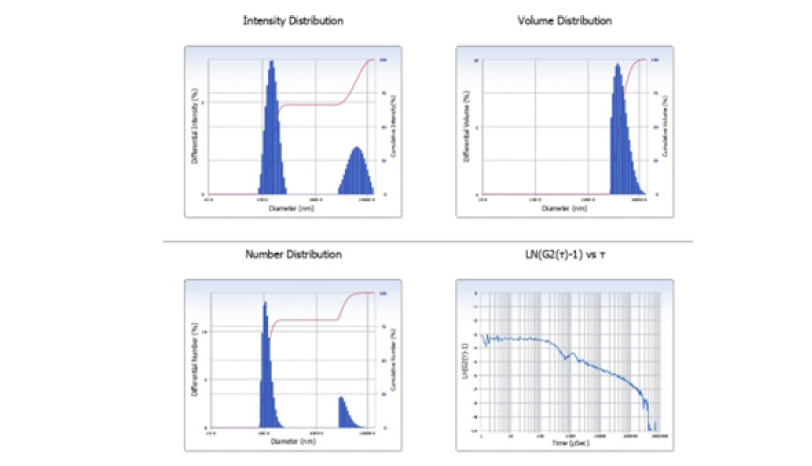
Zeta potential analysis revealed a surface charge of -28.4mV, signifying robust colloidal stability within an aqueous suspension. This observed negative charge is attributed to the deprotonation of functional groups, such as carboxyl and phenolic moieties, present on the biochar surface [48]. The product’s hydrodynamic particle size, determined via dynamic light scattering, was approximately 98.6nm, substantiating its nanoscale characteristics. Its moderate size and considerable stability render it amenable to aqueous dispersion and practical use in contaminant adsorption [49]. The mobility related to the zeta potential was -2.21μm·cm/V·s, further supporting electrostatic repulsion among particles and stable suspension behavior (Figure 8).
Figure 8:EOS plot of zeta potential distribution.
The examination of coconut shell nanobiochar via Electrophoretic Mobility analysis indicates a zeta potential distribution concentrated around 0mV. This suggests that, under the tested conditions, the nanobiochar particles possess a near-neutral surface charge. Such a low zeta potential implies insufficient electrostatic repulsion between particles, which can lead to aggregation and consequently reduce colloidal stability when dispersed. This finding is consistent with the observed pHdependent adsorption of methylene blue. Specifically, at lower pH values, the nanobiochar surface becomes protonated and positively charged, promoting electrostatic repulsion with cationic dye molecules. However, at neutral pH, the surface acquires a more negative charge, enhancing electrostatic attraction and maximizing adsorption efficiency. Therefore, the zeta potential distribution data strongly supports the influence of surface charge characteristics on the adsorption dynamics between the nanobiochar and dye molecules (Figure 9).
Figure 9:Zeta potential distribution of the sample.
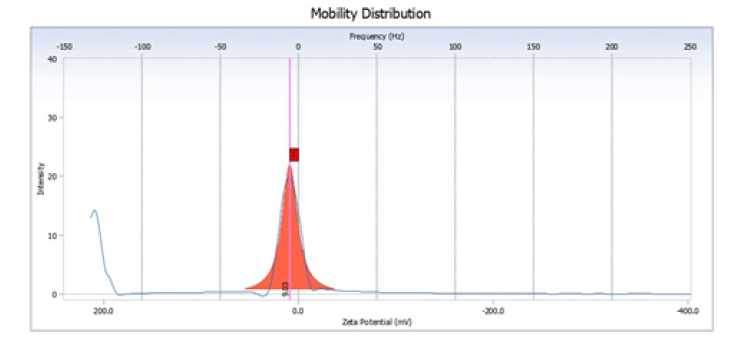
The electrophoretic mobility distribution of coconut shell nanobiochar is characterized by a sharp, narrow peak centered near 0mV. This indicates that the particles possess a nearly neutral surface charge and exhibit very low electrophoretic mobility. The near-zero zeta potential suggests that electrostatic repulsion is limited, leading to poor colloidal stability and a tendency for particle aggregation in suspension. In conjunction with the EOS plot results, it is evident that the nanobiochar’s surface charge is highly sensitive to the surrounding pH, which directly influences its adsorption behavior. At acidic pH levels, surface protonation reduces dye uptake due to electrostatic repulsion with cationic methylene blue. Conversely, at neutral pH, deprotonation results in a negatively charged surface that enhances electrostatic attraction and maximizes adsorption efficiency. Therefore, both EOS and mobility distribution analyses underscore the critical role of surface charge in governing the adsorption performance of coconut shell nanobiochar (Figure 10).
Figure 10:DLS particle size distribution of coconut shell nanobiochar.
Dye removal.
Coconut shell-based nanobiochar, produced through hightemperature pyrolysis of unmodified coconut shells, presents itself as a cost-effective and environmentally benign adsorbent for water treatment. This material’s extensive surface area, sophisticated porous structure, and rich array of surface functional groups enable the effective elimination of contaminants from water. Specifically, CS-NBC exhibits a significant capacity for adsorbing methylene blue, a prevalent cationic dye utilized in the textile sector, achieving up to 98% removal at a neutral pH within 120 minutes. This performance highlights its potential for the remediation of industrial wastewater polluted with organic dyes. The adsorption process is primarily driven by π-π stacking interactions and electrostatic forces between the dye molecules and the negatively charged nanobiochar surface [50,51]. Consistent with prior research, these outcomes corroborate biochar’s capacity to adsorb a range of colorants, including Congo red, rhodamine B, and malachite green [52]. The interaction of methylene blue dye with lignocellulosic biomass is governed by several adsorption mechanisms. These encompass π-π stacking between the aromatic systems of lignin and MB, electrostatic forces between the cationic dye and the biomass’s anionic functional groups, hydrogen bonding between MB molecules and hydroxyl or carboxyl groups (-OH, -COO-), and van der Waals forces that contribute to stabilization. The synergistic effect of these interactions enhances the affinity of MB for lignocellulosic materials, thereby demonstrating their potential as economical and sustainable adsorbents for removing dyes from wastewater [53]; (Figure 11).
Figure 11:Adsorption of methylene blue dye on coconut shell-derived nanobiochar (CS-NBC).
Figure 12:Effect of pH on methylene blue dye removal efficiency using coconut shell nanobiochar.
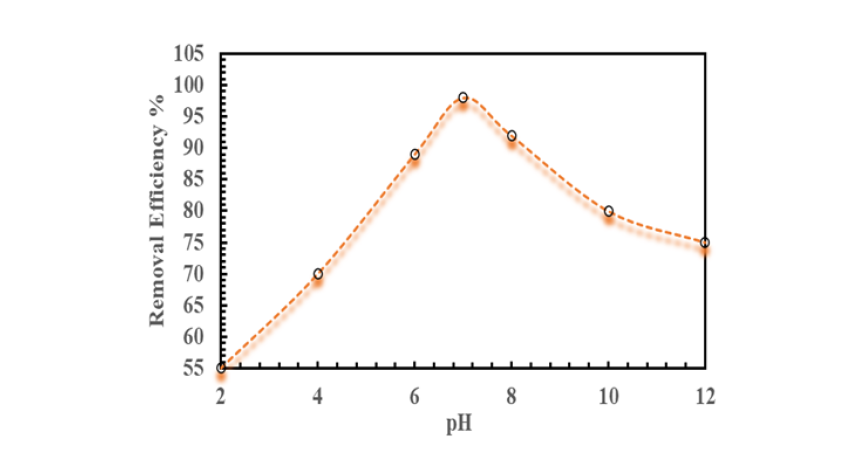
The capacity of coconut shell nanobiochar to adsorb methylene blue dye is significantly affected by the pH of the solution. This is attributed to the influence of pH on both the adsorbent’s surface charge and the ionization state of the dye molecules. Under acidic conditions, the adsorption efficiency is reduced because the protonation of surface functional groups results in a positively charged biochar surface, which electrostatically repels the cationic methylene blue molecules, thereby hindering adsorption. Conversely, adsorption capacity notably improves in near-neutral pH conditions, reaching its peak at pH 7. At this pH, the nanobiochar surface develops a negative charge, promoting electrostatic attraction of methylene blue cations. Furthermore, π-π interactions between the aromatic structures of the biochar and the methylene blue molecules contribute to enhanced binding. However, under alkaline conditions, a gradual decrease in removal efficiency is observed, as excess hydroxyl ions (OH-) compete for adsorption sites and diminish the electrostatic attraction between the dye and the adsorbent. These observations align with previous research indicating that nanobiochars achieve maximal dye removal at neutral pH, owing to the synergistic effects of surface charge characteristics and molecular interactions [54]; (Figure 12).
Regeneration and reusability
Initial regeneration experiments indicate that CS-NBC retains a significant portion of its adsorption capacity after three adsorptiondesorption cycles when using either ethanol or dilute acid washes. This durability suggests its suitability for cost-effective, repeated use in water treatment. Additionally, its thermal stability enables reuse following mild regeneration methods [55].
Conclusion
This research successfully synthesized nanobiochar from coconut shells using controlled pyrolysis, establishing its suitability for adsorption due to its porous architecture, extensive surface area, and characteristic functional groups. The material’s structural and chemical attributes, confirmed through rigorous characterization, were found to be crucial for efficient dye adsorption. Experimental results indicated that the synthesized coconut shell-based nanobiochar achieved high removal rates for methylene blue dye in wastewater treatment, primarily through mechanisms involving π-π interactions, electrostatic attraction, and the nanobiochar’s mesoporous structure. Consequently, CS-NBC emerged as a costeffective, environmentally benign, and sustainable adsorbent derived from agricultural waste, presenting a viable strategy for mitigating dye pollution in wastewater. Its demonstrated adsorption capabilities highlight significant potential for widespread environmental applications, thereby contributing to both waste valorization and the management of water contamination.
Acknowledgment
This research was completed with the generous support and expert supervision of Dr. Santosh Narayan Chadar, to whom the author extends sincere appreciation. Furthermore, the author acknowledges the essential infrastructure and assistance provided by Government Auto Girls P.G. College, Sagar, which were instrumental in the successful execution of this study.
References
- Inyang M, Dickenson E (2015) The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 134: 232-240.
- Rodríguez-Narváez OM, Peralta-Hernández JM, Goonetilleke A, Bandala ER (2019) Biochar-supported nanomaterials for environmental applications. Journal of Industrial and Engineering Chemistry 78: 21-23.
- Sun L, Chen D, Wan S, Yu Z (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus sawdust modified with citric, tartaric, and acetic acids. Bioresource Technology 198: 300-308.
- Tang L, Yu J, Pang Y, Zeng G, Deng Y, et al. (2017) Sustainable, efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chemical Engineering Journal 336: 160-169.
- Khan N, Chowdhary P, Gnansounou E, Chaturvedi P (2021) Biochar and environmental sustainability: Emerging trends and techno-economic perspectives. Bioresource Technology 332:
- Kumar M, Xiong X, Wan Z, Sun Y, Tsang DCW, et al. (2020) Ball milling as a mechanochemical technology for the fabrication of novel biochar nanomaterials. Bioresource Technology 312:
- Ahuja R, Kalia A, Sikka R, Chaitra P (2022) Nano modifications of biochar to enhance heavy metal adsorption from wastewater: A review. ACS Omega 7(50): 45825-45836.
- Zhao C, Wang B, Theng BKG, Wu P, Liu F, et al. (2021) Formation and mechanisms of nano-metal oxide-biochar composites for pollutants removal: A review. Science of the Total Environment 767:
- Wang Y, Chen L, Zhu Y, Fang W, Tan Y, et al. (2024) Research status, trends, and mechanisms of biochar adsorption for wastewater treatment: A scientmetric review. Environmental Sciences Europe 36(1): 25.
- Anderson N, Gu H, Bergman R (2021) Comparison of novel biochars and steam-activated carbon from mixed conifer mill residues. Energies 14(24):
- Faizan M, Alam P, Kumari A, Suresh G, Sharma P, et al. (2024) Unraveling the nano-biochar-mediated regulation of heavy metal stress tolerance for sustaining plant health. Plant Stress
- Zhu L, Zhao N, Tong L, Lv Y, Li G (2018) Characterization and evaluation of surface-modified materials based on porous biochar and its adsorption properties for 2,4-dichlorophenoxyacetic acid. Chemosphere 210: 734-744.
- Hidayat H, Rauf A, Sabrina T, Jamil A (2018) Potential of several biomass species as biochar for heavy metal adsorbent. Journal of Asian Scientific Research 8(11): 293-300.
- Naghdi M, Taheran M, Kaur Brar S, Rouissi T, Verma M, et al. (2017) A green method for production of nanobiochar by ball milling: Optimization and characterization. Journal of Cleaner Production 164:
- Senadheera SS, Gupta S, Wei Kua H, Hou D, Kim S, et al. (2023) Application of biochar in concrete - A review. Cement and Concrete Composites 143:
- Ajien A, Idris J, Sofwan NM, Husen R, Seli H (2022) Coconut shell and husk biochar: A review of production and activation technology, economic, and financial aspects, and application. Waste Management & Research: The Journal for a Sustainable Circular Economy 41(1):
- Kujawska J, Wasąg H (2021) Biochar: A low-cost adsorbent of methylene blue from aqueous solutions. Journal of Physics: Conference Series 1736(1):
- Vijayaraghavan K (2019) Recent advancements in biochar preparation, feedstocks, modification, characterization, and future applications. Environmental Technology Reviews 8(1):
- Tang J, Zhu W, Kookana RS, Katayama A (2013) Characteristics of biochar and its application in the remediation of contaminated soil. Journal of Bioscience and Bioengineering 116(6):
- Pariyar P, Kumari K, Jain MK, Jadhao P (2020) Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural applications. Science of the Total Environment 713:
- Sun X, Atiyeh HK, Li M, Chen Y (2019) Biochar facilitated bioprocessing and biorefinery for the production of biofuel and chemicals: A review. Bioresource Technology 295: 122252.
- Jiang F, Li F, Zimmerman AR, Yu Z, Ji L, et al. (2023) Remarkable synergy between sawdust biochar and attapulgite/diatomite after co-ball milling to adsorb methylene blue. RSC Advances 13(21): 14384.
- Jindo K, Mizumoto H, Sawada Y, Sánchez-Monedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11(23): 6613.
- Ding Y, Liu Y, Liu S, Huang X, Li Z, et al. (2017) Potential benefits of biochar in agricultural soils: A review. Pedosphere 27(4): 645-661.
- Khater ES, Bahnasawy A, Hamouda R, Sabahy A, Abbas W, et al. (2024) Biochar production under different pyrolysis temperatures with different types of agricultural wastes. Scientific Reports 14(1).
- Nartey OD, Zhao B (2014) Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Advances in Materials Science and Engineering 2014: 715398.
- Peterson SC, Jackson MA, Appell M (2013) Biochar: Sustainable and versatile. Advances in Applied Nanotechnology for Agriculture 1143: 193-205.
- Sun Y, Gao B, Yao Y, Fang J, Zhang M, et al. (2013) Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chemical Engineering Journal 240: 574-578.
- Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, et al. (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technology 118: 536-544.
- Zhang M, Gao B, Yao Y, Xue Y, Inyang M (2012) Synthesis of porous MgO-biochar nanocomposites for the removal of phosphate and nitrate from aqueous solutions. Chemical Engineering Journal 210: 26-32.
- Inyang M, Gao B, Yao Y, Xue Y, Zimmerman A, et al. (2016) A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Critical Reviews in Environmental Science and Technology 46(4): 406-433.
- Mohan D, Sarswat A, Ok YS, Pittman CU (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low-cost, and sustainable adsorbent - A critical review. Bioresource Technology 160: 191-202.
- Tan X, Liu Y, Zeng G, Wang X, Hu X, et al. (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125: 70-85.
- Wang S, Gao B, Li Y, Creamer AE, He F, Zimmerman AR (2017) Biochar derived from waste biomass as an alternative adsorbent and renewable energy: A critical review. Renewable and Sustainable Energy Reviews 79: 576-597.
- Leng L, Huang H, Li H, Li J, Zhou W, Zhang W (2021) Biochar stability: A review of physicochemical influences and mechanisms. Bioresource Technology 341: 125803.
- Abbas M, Trari M (2024) Adsorption behavior of methylene blue onto activated coconut shells: Kinetic, thermodynamic, mechanism, and regeneration of the adsorbent. Dose-Response 22(4).
- Abubakar AM, Nazar N, Yusuf AA, Idama EA, Arowo MN, et al. (2025) Development of low-cost adsorbents from coconut shell for energy-efficient dye removal from laboratory effluent discharge. Measurement: Energy 7:
- Del Grosso M, Cutz L, Tiringer U, Tsekos C, Taheri P, et al. (2022) Influence of indirectly heated steam-blown gasification process conditions on biochar physico-chemical properties. Fuel Processing Technology 235:
- Zhang M, Gao B, Yao Y, Xue Y, Inyang M (2012) Synthesis of porous MgO-biochar nanocomposites for the removal of phosphate and nitrate from aqueous solutions. Chemical Engineering Journal 210: 26-32.
- Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, et al. (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technology 118: 536-544.
- Leng L, Huang H, Li H, Li J, Zhou W, et al. (2021) Biochar stability: A review of physicochemical influences and mechanisms. Bioresource Technology 341: 125803.
- Tan X, Liu Y, Zeng G, Wang X, Hu X, et al. (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125: 70-85.
- Yuan P, Wang J, Pan Y, Shen B, Wu C (2019) Review of biochar for the removal of pollutants from aqueous solutions. Chemosphere 202: 644-658.
- Wang S, Gao B, Li Y, Creamer AE, He F, et al. (2017) Biochar derived from waste biomass as an alternative adsorbent and renewable energy: A critical review. Renewable and Sustainable Energy Reviews 79: 576-597.
- Yang Y, Zhang M, Wen Y, Gao B (2019) Characterization of biochar derived from coconut shells for efficient removal of methylene blue dye. Environmental Science and Pollution Research 26(22): 22458–22467.
- Inyang M, Gao B, Yao Y, Xue Y, Zimmerman A, et al. (2016) A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Critical Reviews in Environmental Science and Technology 46(4): 406-433.
- Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, et al. (2014) Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 99: 19-33.
- Rajapaksha AU, Vithanage M, Ahmad M, Seo DC, Ok YS (2015) Dissolution of biochars induced by changes in pH, ionic strength, and sorbate-sorbent interactions. Chemosphere 142: 254-261.
- Luong THV, Li CZ, Zhu Z (2020) Surface charge and colloidal stability of biochars derived from different biomass feedstocks and pyrolysis temperatures. Environmental Technology & Innovation 19: 100839.
- Mohan D, Sarswat A, Ok YS, Pittman Jr CU (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low-cost, and sustainable adsorbent - A critical review. Bioresource Technology 160: 191-202.
- Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochemistry 34(5): 451-465.
- Ghosh I, Bar N, Das SK (2024) Methylene blue dye removal by adsorption with coconut coir and acid-treated forms: adsorption-desorption study, MPR, and modeling using artificial intelligence. Journal of Dispersion Science and Technology pp. 1-26.
- Zhou Y, Lu J, Zhou Y, Liu Y (2019) Recent advances for dyes removal using novel adsorbents: A review. Environmental Pollution 252(Pt A): 352-365.
- Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal-A review. Journal of Environmental Management 90(8): 2313-2342.
- Xu J, Fu M, Ma Q, Zhang X, You C, et al. (2023) Modification of biochar by phosphoric acid via wet pyrolysis and using it for the adsorption of methylene blue. RSC Advances 13(22): 15327-15333.
© 2025 © Santosh Narayan Chadar. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






