- Submissions

Full Text
Research & Development in Material Science
Glimpses into the Dynamics and Static Ever Evolution in Ge-Se Glasses
Deepak Sharma*
SCRIET, C.C.S. University Meerut, India
*Corresponding author:Deepak Sharma, SCRIET, C.C.S. University Meerut-250001, India.Thiruvananthapuram, India
Submission: August 22, 2025;Published: September 03, 2025

ISSN: 2576-8840 Volume 22 Issue 2
Abstract
The article investigates the evolution of glass transition temperature (Tg) with variation of chemical compositions in Ge-Se chalcogenide glasses, focusing on the correlation between dynamical parameter Tg and static parameter compactness (δ). We experimentally measure Tg for various Ge-Se compositions and explore different fitting models. The author initially fit the log (Tg) against the average coordination number. This paper provides valuable insights into the kinetic and static properties of Ge-Se glasses, showcasing an innovative application of the modified Gibbs-Di-Marzio equation. Through this analysis, we are able to ascertain that the constant β in the Gibbs-Di-Marzio equation is dependent on the ratio of two temperatures and that the value of β (0.74) derived from the fit, while the value of β = 0.72134 derived from the theory for the Ge-Se binary system. We are extending this empirical Gibbs-Di-Marzio form in another way to enrich our scientific research for Ge-Se binary system.
Keywords:Chalcogenide glasses; Glass transition temperature; Compactness; Chemical compositions; Glass dynamics
Introduction
Chalcogenide glasses have considerable interest due to potential applications in the advancement of technology. Their promising applications are in the area of phase change memory devices in computing, fiber laser, as well as in the optical fiber communications. Additionally, glasses don’t possess long range orders. Alloys of group IV and group VI based chalcogenide glasses allow the modification significantly in their structural and thermal properties. Hence, the composition dependent study of thermal properties, such as glass transition temperature is of great importance to understand the structural relaxation phenomena and viscous nature of the glass, to improve their applications in technology. Ge-Se is a typical binary chalcogenide system which is widely studied. Beyond its dynamical properties its static properties are significantly important. Understanding thermal properties such as glass transition and crystallization is important to improve switching characteristic for phase change memory devices. Thus, chemical compositions-based variations in static and dynamic property correlation is not studied yet. This system is, therefore, ideal for analysis and correlation studies between dynamic and static property is to address in this paper. The author initially fit log (Tg) against the average coordination number
Experimental
Bulk glassy compositions were prepared by taken elemental Germanium (Ge) and elemental selenium (Se) 5N purity. In a batch 10gm of compositions were prepared. Weighed chunks of elemental (Ge) and Selenium were crushed in a pestle mortar. These crushed powders were mixed up and transferred into silica ampoule. Sample powders in the ampoule were evacuated at a pressure of 10-6 Torr by using diffusion pump. Hence, silica ampoules were sealed. Sealed ampoules were kept into rotating furnace. Temperature of the furnace was gradually increased up to 950 ℃. This temperature was monitored at the glass ampoule with the help of thermocouple tip. After 24 hours, melt was rotated for two days at this temperature to ensure make it homogenous. After two days sample was quenched into mixture of ice water in a bucket. Melt quenched samples were retrieved from the ampoule. Samples were annealed for seven days and after that thermal measurements were performed by Modulated Differential Scanning Calorimeter (MDSC) TA Instrument 2910. Measurements were performed from room temperature by ramp the temperature, at a heating rate of 5 ℃/min. Glassy nature of the sample was characterized and glass transition temperature was determined.
Results and Discussion
Figure 1 shows a typical thermal curve for the glassy Ge20Se80 composition that was produced by using an MDSC instrument. At the glass transition temperature, an endothermic peak for structural relaxation was observed, which is also displayed in Figure 1. The glass transition temperature was estimated from the heat capacity versus temperature curve shown in Figure 1.
Figure 1:Structural relaxation in non-reversing heat flow and glass transition in heat capacity curve.
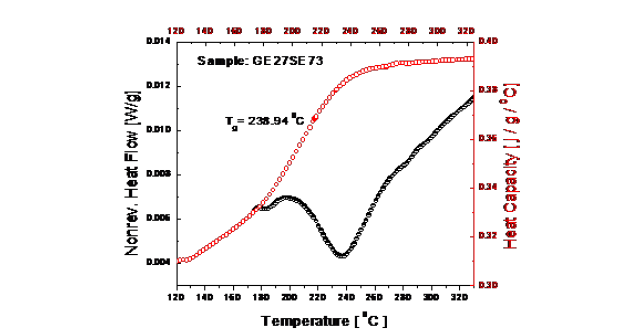
The glass transition temperatures values were obtained
from different chemical compositions by doping germanium
into selenium matrix. Coordination number of Germanium (Ge)
and Selenium (Se) are 4 and 2, respectively. The Composition
dependence of the thermal parameter Tg in terms of average
coordination number
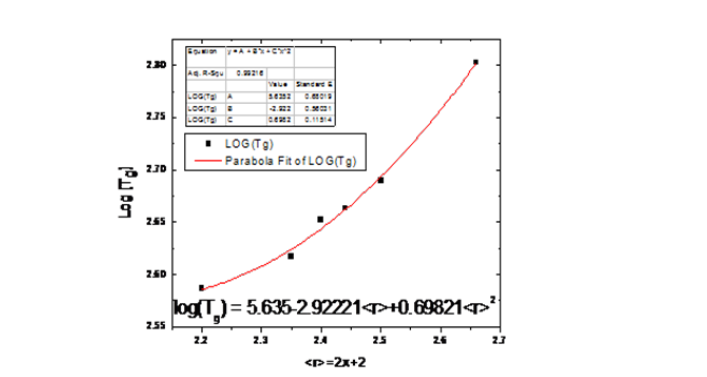
To refine this fit on the experimental data, we found a new
phase of their relationship with equation y = A +Bx+Cx2 where x
represents the average coordination number, now log(Tg) = 5.635-
2.92221
Figure 2:Variation of log (Tg) versus
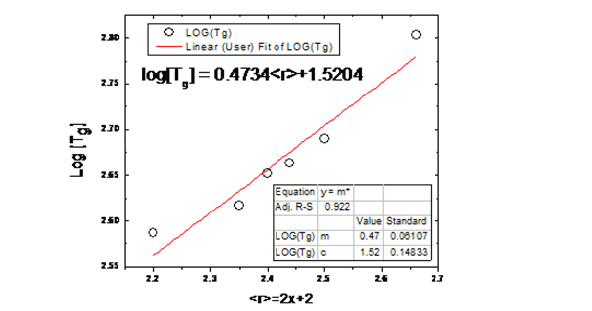
Figure 3:log (Tg) versus < r > red line fitted on experimental data with more accuracy.
It is understandable that glass transition Tg is mainly related to the network rigidity, the number of bonds per atom, and the bond energies between the atoms in the network [2]. Doping Germanium into polymeric selenium chains increases the network rigidity, as evident also from the dynamic /kinetic glass transition values, glass transition temperature increases with germanium doping up to chemical threshold composition Ge33 Se67. Hence, the glass transition must be proportional to the overall mean bond energy < E > of the glassy sample. Many semi-empirical relationships have been proposed to fit the measured Tg for particular system [3]. Particularly, Gibbs and DiMarzio [4] and DiMarzio [5] have shown that glass transition temperature is given by
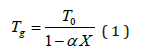
where T0 is the glass transition temperature of non -crossedlink initial polymeric chain, X is the cross linked density and α is a universal constant. Equation (1) is purely on the basis of thermodynamic considerations and therefore, it is suited for the application of chalcogenide glasses.
Sreeram et al. [6] adapted Gibbs DiMarzio equation by
redefining the cross-linking density, X, as being equal to the average
coordination number
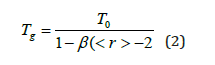
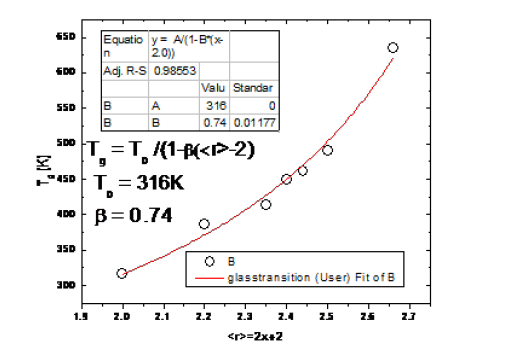
Equation (2) is physically significant when the values of must lie between 0 and 1. In this work, we demonstrate that for the glassy Ge-Se system, the modified Gibbs Di-Marzio equation describes the evolution of the glass transition as a function of the average coordination number, as shown in Figure 4. This equation is in good agreement with the experimental data, and we have estimated the value of T0 = 316K and the constant β = 0.74, so the modified equation for Ge-Se system is written as follows:
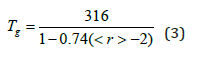
Figure 4:Modified Gibbs- Di-Marzio fit red line on experimental data with constant β = 0.74.
Sreeram et al. [6] investigation of multi-component chalcogenide systems from pure vitreous selenium β is a system dependent parameter and is given by:
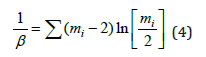
Here we computed the value of β from the equation (4), the calculated value of β is 0.72134 for the Ge-Se binary system. The theoretical and experimental aspects of the fitted data are in very good agreement with the modified Gibbs-Di-Marzio equation. However, the data presented by group S. K. Tripathi et al. [7] in the Se-Te-Sb system, the theoretical values of modified Gibbs -Di- Marzio law did not agree with the experimental values. In this article, we also extended the Gibbs-Di Marzio equation proposing a modification for the Ge-Se system. Suppose we don’t have data for pure selenium and I have one composition close to pure selenium in Ge-Se binary system, namely Ge2Se98, then we can replace the glass transition temperature of this composition in place of pure selenium. In this case we can extend the modified Gibbs- Di -Marzio equation, which can be written as:
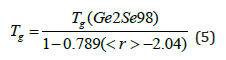
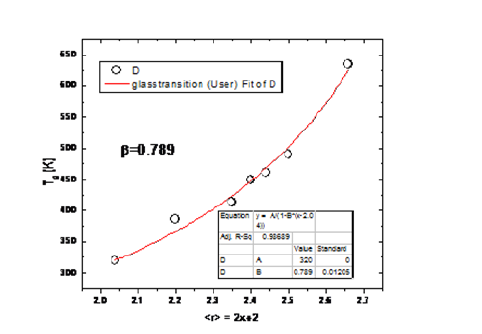
This is shown in Figure 5 with a β value 0.789, which fits very well to the equation (5) for the entire series of the compositions; but the cross linking for Ge2 Se98 composition is very small, so the accuracy of the experimental and theoretical results is good. This clearly indicates that T0 can be easily replaced by composition with very less cross-linking glass transition temperature, which is applicably suitable for the Ge-Se glass series.
Figure 5:Extended Modified Gibbs-Di-Marzio fit Tg = TGe2 Se98/(1-β(
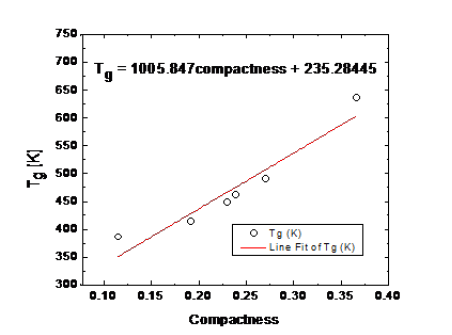
We estimated the modified compactness [8] for Ge-Se glasses and are interested in the cross correlation between the variation of the glass transition temperature (dynamic quantity) and the obtained (static) compactness. The quasi-linear fitting of dynamic and static cross-correlation is shown in Figure 6, the fitting relationship is expressed as Tg = 1005.847 ×compactness + 235.28445. The quasi-linear fitting does not show good accuracy for all data points. To overcome this problem, we tried to fit the equation y = A+ Bx + Cx2 for this cross-correlation, we got a good fitting result as:
Tg=410.0493-598.237×compactness+3314.973× (compactness)2, as shown in Figure 7.
Figure 6:Relationship between glass transition and static compactness by fitted red line.
Conclusion
We are confident that our insights significantly enrich our scientific research and latest advancement in the technological applications; not only in the field of Ge-Se system but it also suggested to explore other multi-component chalcogenide glass system to testify the approach.
References
- Tanaka K (1985) Glass transition of covalent glasses. Solid State Communication 54:
- Harikuttan Unnithan C, Pardeep P, Jayakumar S (2003) Doping effect of copper in germanium tellurium semiconducting glass system. J Phys Chem Solids 64(4): 707-709.
- Tichy L, Ticha H (1995) Covalent bond approach to the glass-transition temperature of chalcogenide glasses. J Non-Cryst Solids 189:
- Gibbs JH, DiMarzio (1958) Nature of the glass transition and the glassy state. J Chem Phys 28:
- DiMarzio EA (1964) On the second-order transition of rubber. J Res Nat Bur Stand 68A(6): 611-617.
- Sreeram AN, Swiller DR, Varshneya AK (1991) Gibbs-DiMarzio equation to describe the glass transition temperature trends in multicomponent chalcogenide glasses. J Non-Cryst Solids 127:
- Balbir SP, Neha, Nagesh T, Tripathi SK (2014) Estimation of Tg for Se-Te-Sb system using modified Gibbs-Dimarzio law. International Journal of Engineering Research and Technology 3(9): 910-912.
- Deepak S (2024) Theoretical model and experimental analysis for optical band gap. Res Dev Material Sci 21(2): 2513-2516.
© 2025 © Deepak Sharma. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






